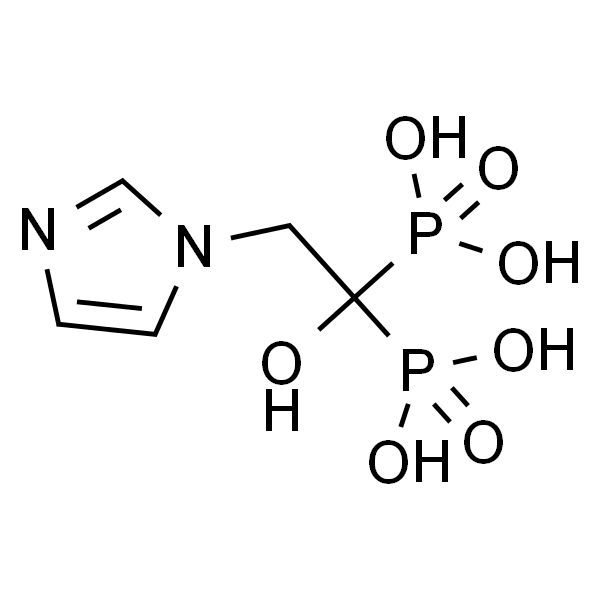
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Supply with High Purity and Stable Quality Chemical Name: Zoledronic Acid CAS: 118072-93-8 Potent Farnesyl Diphosphate (FPP) Synthase Inhibitor API High Quality, Commercial Production| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification | IR Spectrum of the sample corresponds to that of the reference standard |
| pH | 2.0~4.0 |
| Solubility | Sparingly Soluble in 0.1N Sodium Hydroxide Solution, Slightly Soluble in Water and 0.1N Hydrochloric Acid, Practically Insoluble in Ethanol |
| Related Substances | |
| Any Individual Impurity | ≤0.10% |
| Total Impurities | ≤0.30% |
| Loss on Drying | 5.0%~7.0% |
| Chloride | ≤0.02% |
| Phosphite | ≤0.50% |
| Phosphate | ≤0.50% |
| Heavy Metals | ≤10ppm |
| Purity | ≥99.7% |
| Test Standard | Enterprise Standard |
| Usage | API, FPP Synthase Inhibitor, Hypercalcemia of Malignancy (HCM) |
Description:
Specifications:
Package & Storage:
| Chemical Name | Zoledronic Acid |
| Synonyms | ZOL 446, ZA, Zoledronate, CGP-4244, GP42446A, Zometa, Zomera; [1-Hydroxy-2-(1H-imidazol-1-yl)-ethylidene]bisphosphonic acid |
| CAS Number | 118072-93-8 |
| CAT Number | RF-API90 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C5H10N2O7P2 |
| Molecular Weight | 272.09 |
| Melting Point | 193.0~204.0℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Zoledronic Acid (CAS 118072-93-8) is a potent bisphophonate farnesyl diphosphate (FPP) synthase inhibitor (IC50=20 nM). Inhibits osteoclast-mediated bone resorption. Also inhibits Ras signaling and tumor growth, and induces apoptosis in pancreatic cancer cells. Reverses epithelial-mesenchymal transition and inhibits breast cancer cell renewal via inactivation of NF-κB. Zoledronic acid was approved by the U.S. FDA in 2001 for the treatment of hypercalcemia of malignancy, a metabolic complication that can be life-threatening. Hypercalcemia of malignancy can occur in up to 50% of patients diagnosed with advanced breast cancer, multiple myeloma, and nonsmall cell lung cancer. This condition arises when chemical moieties produced by the tumor cause overstimulation of osteoclasts. When there is an increase in bone degradation, there is a concomitant release of calcium into the plasma. When serum concentrations of calcium rapidly elevate, the kidneys are unable to handle the overload, and hypercalcemia results. Zoledronic acid effectively decreases plasma calcium concentrations via inhibition of bone resorption (inhibition of osteoclastic activity and induction of osteoclast apoptosis). Additionally zoledronic acid has been approved by the U.S. FDA for the treatment of multiple myeloma and bone metastases associated with solid tumor-based cancers (e.g., prostrate and lung).