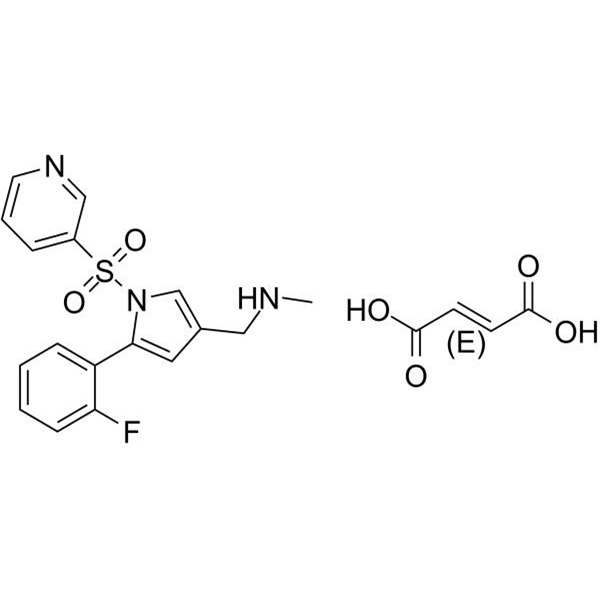
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture.Supply Vonoprazan Fumarate and Related Intermediate 5-(2-Fluorophenyl)pyrrole-3-Carboxaldehyde CAS 881674-56-2 Pyridine-3-Sulfonyl Chloride CAS 16133-25-8 Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 881681-01-2| Item | Specifications |
| Appearance | White or Almost-White Crystalline Powder |
| Identification 1 H-NMR | Consistent with the Standard |
| Identification IR | The IR Absorption spectrum of the sample concordant with reference standard |
| Identification HPLC | The retention time of the sample concordant with reference standard |
| Water Content | ≤0.50% |
| Residue on Ignition | ≤0.20% |
| Heavy Metals (as Pb) | ≤20ppm |
| Related Substances | |
| Impurity A | ≤0.20% |
| Impurity B | ≤0.15% |
| Impurity C | ≤0.15% |
| Any Other Impurity | ≤0.10% |
| Total Impurities | ≤1.0% |
| Residual Solvents | |
| Methanol | ≤0.30% |
| Ethanol | ≤0.50% |
| Ethyl Acetate | ≤0.50% |
| Assay / Analysis Method | 98.0%~102.0% (By HPLC) |
| Test Standard | Enterprise Standard |
| Usage | API |
Description:
Specifications:
Package & Storage:
| Chemical Name | Vonoprazan Fumarate |
| Synonyms | TAK-438 |
| CAS Number | 1260141-27-2; 881681-01-2 |
| CAT Number | RF-API99 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C21H20FN3O6S |
| Molecular Weight | 461.463 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Vonoprazan Fumurate (TAK-438) (CAS: 1260141-27-2; 881681-01-2), a proton pump inhibitor (PPI), is a potent and orally active potassium-competitive acid blocker (P-CAB), with antisecretory activity. Vonoprazan Fumarate inhibits H+,K+-ATPase activity in porcine gastric microsomes with an IC50 of 19 nM at pH 6.5. Vonoprazan Fumarate (Takecab), discovered and developed by Takeda and Otsuka, was approved by the PMDA of Japan in December 2014. Vonoprazan Fumarate is mainly used for the treatment of helicobacter pylori infection, gastroesophageal reflux, peptic ulcer, duodenal ulcer, esophagitis, gastric ulcer and other gastric acid related diseases. The product has a strong, lasting inhibition of gastric acid secretion. At the treatment dose, Vonoprazan Fumarate has little effect on other enzymes and little effect on physiological functions of the body. It is safe and more tolerable.