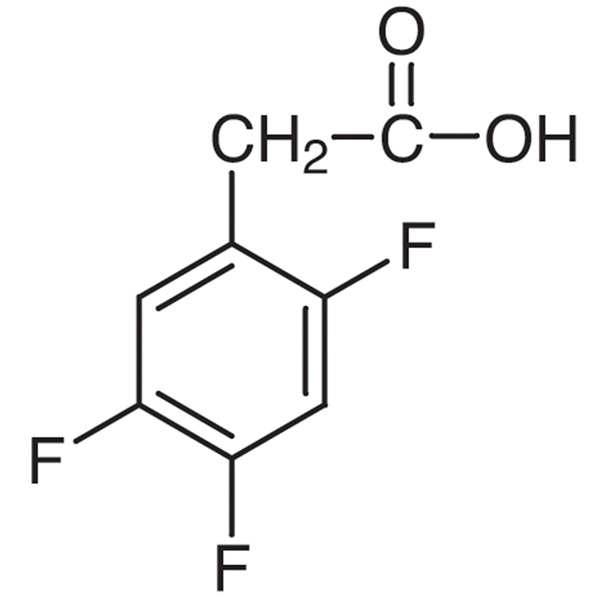
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Supply Sitagliptin Phosphate Monohydrate Related Intermediates: Sitagliptin API CAS 486460-32-6 Sitagliptin Phosphate Monohydrate API CAS 654671-77-9 2,4,5-Trifluorophenylacetic Acid CAS 209995-38-0 Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Butyric Acid CAS 486460-00-8 Sitagliptin Triazole Hydrochloride CAS 762240-92-6 Sitagliptin Phosphate Monohydrate Intermediate CAS 486460-21-3| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity / Analysis Method | ≥99.5% (GC) |
| Moisture (K.F) | ≤0.50% |
| Residue on Igniton | ≤0.50% |
| Total Impurities | ≤0.50% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Sitagliptin Phosphate Monohydrate (CAS: 654671-77-9) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 2,4,5-Trifluorophenylacetic Acid |
| CAS Number | 209995-38-0 |
| CAT Number | RF-PI1191 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H5F3O2 |
| Molecular Weight | 190.12 |
| Melting Point | 121.0 to 125.0℃ |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2,4,5-Trifluorophenylacetic Acid (CAS: 209995-38-0) is an intermediate of Sitagliptin Phosphate Monohydrate (CAS: 654671-77-9). Sitagliptin Phosphate (STG) is used to treat DM type 2 because it improves glycemic control by increasing the levels of active incretin hormones, GLP-1 (peptide-1) and GIP (glucose-dependent insulinotropic peptide). The activation of these incretins in β-pancreatic cells causes increased levels of cyclic adenosine monophosphate (cAMP) and intracellular calcium, with subsequent glucose-dependent insulin secretion (2). This hypoglycemic drug belongs to a new class called dipeptidyl peptidase IV inhibitors. STG was approved by the FDA in 2006.