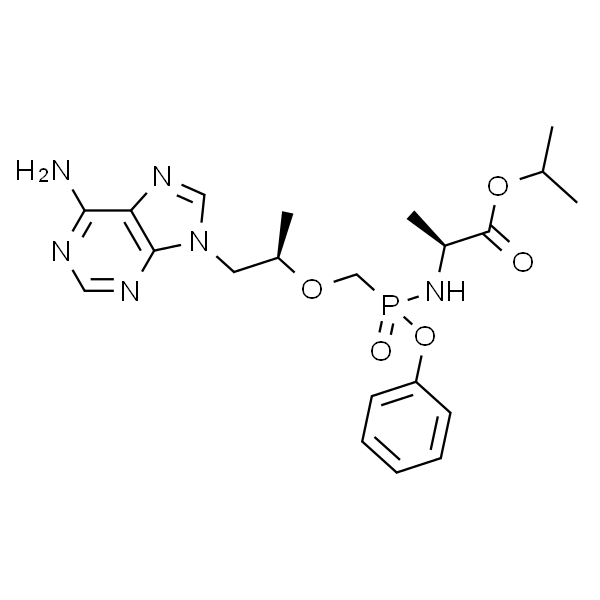
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Commercial Supply Tenofovir Related Intermediates: Tenofovir CAS: 147127-20-6 Tenofovir Disoproxil Fumarate CAS 202138-50-9 Tenofovir Alafenamide Hemifumarate CAS 1392275-56-7 Chloromethyl Isopropyl Carbonate CAS 35180-01-9 Diethyl (p-Toluenesulfonyloxymethyl)phosphonate CAS 31618-90-3 (R)-(+)-Propylene Carbonate CAS 16606-55-6 (R)-9-(2-Hydroxypropyl)adenine CAS 14047-28-0 Diethyl (Hydroxymethyl)phosphonate CAS 3084-40-0 Adenine CAS 73-24-5| Item | Specifications |
| Appearance | White to Almost White Crystals or Crystalline Powder |
| Solubility | Soluble in Dimethylformamide, and Methanol; Sparingly Soluble in Water and THF; Slightly Soluble in Acetonitrile and Insoluble in Dichloromethane and Toluene |
| Identification A | The IR absorption spectra should be consistent with the reference |
| Identification B | The retention time of the major peak of the sample solution in the content determination chromatogram conforms to that of the reference solution |
| Identification C | The Fumaric Acid retention time of the major peak in the content determination chromatogram conforms to the peak of the reference |
| Water Content (by K.F) | ≤1.0% |
| Clarity of Solution | Basically no visible particles in 2% (w/v) of the Methanol Solution clarification |
| Residual Solvent | |
| Acetonitrile | ≤410ppm |
| Ethanol | ≤5000ppm |
| Dichloromethane | ≤600ppm |
| Toluene | ≤890ppm |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤20ppm |
| Related Substances | |
| PMPA | ≤1.0% |
| PMPA Anhydride | ≤0.75% |
| Single Phenyl PMPA | ≤1.0% |
| Any Unspecified Impurity | ≤0.15% |
| Total Impurities | ≤2.0% |
| Isomer | ≤1.0% |
| Content of Fumaric Acid | 9.0%~13.0% (on the anhydrous basis ) |
| Microbial Limits | |
| Total Aerobic Microbial Count | ≤1000CFU/g |
| Total Yeasts and Molds Count | ≤100CFU/g |
| Escherichia Coli | Absent |
| Assay | 98.0%~102.0% (on the anhydrous and solvent-free basis ) |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Tenofovir Alafenamide Hemifumarate |
| Synonyms | TAF; GS-7340 Hemifumarate |
| CAS Number | 1392275-56-7 |
| CAT Number | RF-API91 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C25H33N6O9P |
| Molecular Weight | 592.55 |
| Storage Temperature | Sealed in Dry, Room Temperature |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Tenofovir Alafenamide Hemifumarate (TAF, GS-7340 hemifumarate), CAS 1392275-56-7, is a nucleotide reverse transcriptase inhibitor (NRTIs) and a novel prodrug of Tenofovir (TFV). By blocking reverse transcriptase, TAF prevent HIV from multiplying and can reduce the amount of HIV in the body. Tenofovir alafenamide is a prodrug, which means that it is an inactive drug. In the body, tenofovir alafenamide is converted to Tenofovir diphosphate (TFV-DP). Tenofovir Alafenamide Fumarate was approved in November 2015 for treatment of HIV-1.