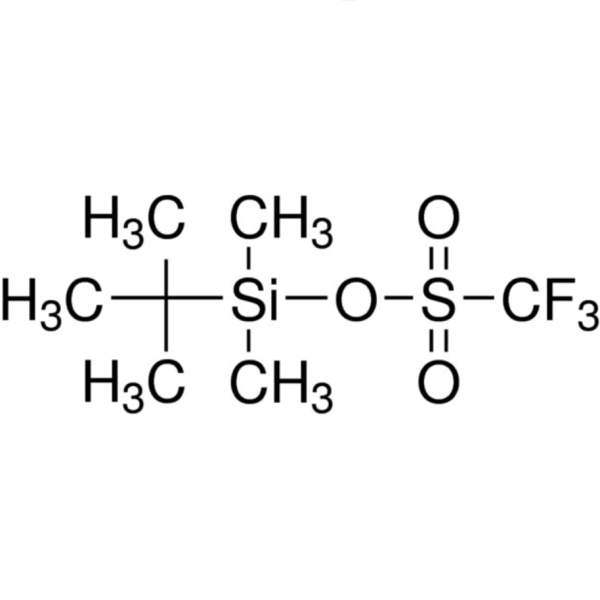Chemical Properties:
Package: Fluorinated Bottle, 25kg/Barrel, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of tert-Butyldimethylsilyl Trifluoromethanesulfonate (CAS: 69739-34-0) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | Colorless to Light Yellow Fuming Liquid |
| Purity / Analysis Method | >98.0% (Neutralization Titration) |
| Refractive Index n20/D | 1.384~1.388 |
| Density (20℃) | 1.148~1.156 |
| Water (K.F) | <0.10% |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | tert-Butyldimethylsilyl Trifluoromethanesulfonate |
| Synonyms | Trifluoromethanesulfonic Acid tert-Butyldimethylsilylester; tert-Butyldimethylsilyl Triflate; TBDMS Triflate; TBS Triflate |
| CAS Number | 69739-34-0 |
| CAT Number | RF-PI2112 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C7H15F3O3SSi |
| Molecular Weight | 264.34 |
| Boiling Point | 65.0~67.0℃/12 mm Hg(lit.) |
| Water Solubility | Decomposes |
| Sensitivity | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: Reacts Rapidly With Moisture, Water, Protic Solvents |
| Hazard Note | Flammable / Corrosive |
| Hazard Class | 3 |
| Packing Group | III |
| HS Code | 29310095 |
| Shelf Life | 60 Months |
| Articles / Brochures | Silicon Compounds |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
tert-Butyldimethylsilyl Trifluoromethanesulfonate (CAS: 69739-34-0) is a highly reactive silylating agent and lewis acid capable of converting primary, secondary and tertiary alcohols to their respectctive TBDMS. TrifluoroMethanesulfonic acid tert-butyldiMethylsilyl ester is also used to covert ketones and lactones into their enol silyl ethers. TBDMS Triflate is used as a highly reactive silylating agent and Lewis acid capable of converting primary, secondary, and tertiary alcohols1b to the corresponding TBDMS ethers, and converting ketones and lactones, into their enol silyl ethers; promoting conjugate addition of alkynylzinc compounds and triphenylphosphine5 to α,β-enones; activation of chromones in [4 + 2] cycloaddition reactions;rearrangement of allylic tributylstannyl silyl ethers; activation of pyridine rings toward Grignard reagents and transalkylation of tertiary amine N-oxides;and transformation of N-t-butoxycarbonyl groups into N-alkoxycarbonyl groups.