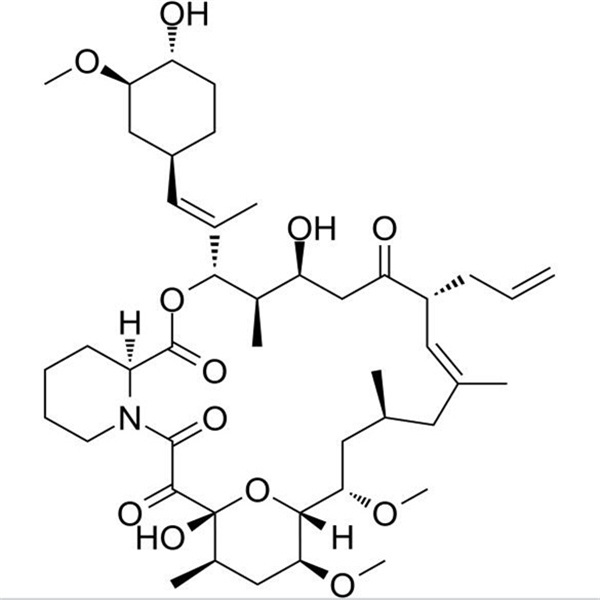
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer with High Purity and Stable Quality Chemical Name: Tacrolimus Synonyms: FK-506; Fujimycin CAS: 104987-11-3 API, High Quality, Commercial Production| Item | Specifications |
| Appearance | Off-White or Pale Yellow Fine Powder, Odourless, Special Sweet Taste |
| Identification | Should be Positive Reaction |
| Clarity | Comply with the Standard |
| pH | 5.0~6.0 |
| Chloride | ≤0.014% |
| Sulphate | ≤0.029% |
| Heavy Metals (Pb) | ≤10ppm |
| Arsenic | ≤0.0002% |
| Moisture (K.F) | ≤8.0% |
| Residue on Ignition | 18.0%~22.0% |
| Assay | ≥72.0% (HPLC, on the dried basis) |
| Test Standard | Enterprise Standard |
| Usage | API |
Description:
Specifications:
Package & Storage:
| Chemical Name | Tacrolimus |
| Synonyms | FK-506; Fujimycin |
| CAS Number | 104987-11-3 |
| CAT Number | RF-API46 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C44H69NO12 |
| Molecular Weight | 804.02 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Tacrolimus (also FK-506 or Fujimycin) is an immunosuppressive drug whose main use is after organ transplant to reduce the activity of the patient's immune system and so the risk of organ rejection. It is also used in a topical preparation in the treatment of severe atopic dermatitis, severe refractory uveitis after bone marrow transplants, and the skin condition vitiligo. Tacrolimus was first extracted from the fermentation broth of Streptomyces tsukuba, a soil microbe found in Tsukuba, Japan. The name tacrolimus is derived by taking the ‘t’ for Tsukuba, the name of the mountain where the soil sample was extracted, ‘acrol’ for macrolide and ‘imus’ for immunosuppressant. Although structurally unrelated to cyclosporin, tacrolimus shows a similar spectrum of immunosuppressive effects to this agent at the cellular and molecular level. Initial studies indicated that tacrolimus was a powerful immunosuppressant, displaying approximately 100-fold greater in vitro potency than cyclosporin in inhibiting T cell activation. Subsequent in vivo studies have shown tacrolimus to be effective both in suppressing spontaneous and experimental autoimmune disease, and in preventing allograft and xenograft rejection in animal models of organ transplantation. Initially, tacrolimus was used for systemic immunosuppression of patients who had undergone allograft transplants to stop them from rejecting their new grafts. Soon, however, through the benefit of the serendipity of science, it was noticed that tacrolimus could produce favorable results in skin disorders in some of the patients who had undergone transplantation. The discovery of tacrolimus has thus lead to greater understanding of skin pathology, for example of atopic dermatitis. Subsequently, other topical applications of tacrolimus were reported and the use of this agent in dermatology is gradually expanding.