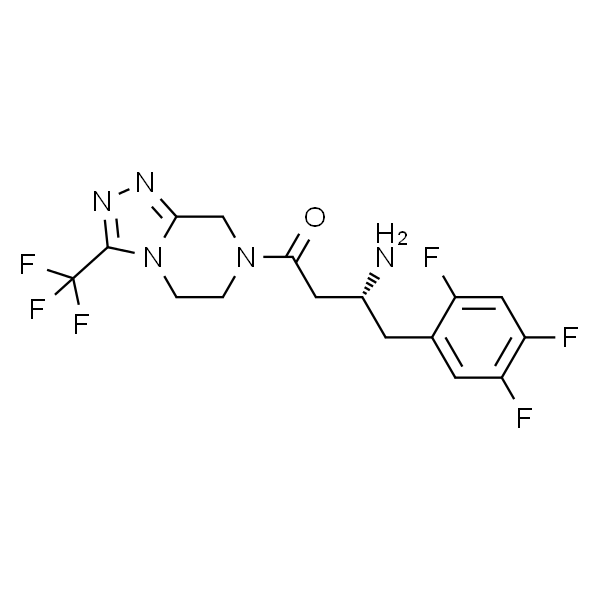
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Supply Sitagliptin Phosphate Monohydrate Related Intermediates: Sitagliptin API CAS 486460-32-6 Sitagliptin Phosphate Monohydrate API CAS 654671-77-9 2,4,5-Trifluorophenylacetic Acid CAS 209995-38-0 Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Butyric Acid CAS 486460-00-8 Sitagliptin Triazole Hydrochloride CAS 762240-92-6 Sitagliptin Phosphate Monohydrate Intermediate CAS 486460-21-3| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification by HPLC | The Retention Time of Sample is Concordant With Reference Standard |
| Loss on Drying | <1.0% |
| Residue on Ignition | <0.20% |
| Related Substances | |
| Any Single Impurity | <0.50% |
| Total Impurities | <1.0% |
| Purity | >99.0% (HPLC) |
| Enantiomeric Purity | <0.50% |
| Heavy Metals | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | API; Type II Diabetes Mellitus |
Description:
Specifications:
Package & Storage:
| Chemical Name | Sitagliptin |
| Synonyms | (3R)-3-Amino-1-[3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one |
| CAS Number | 486460-32-6 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C16H15F6N5O |
| Molecular Weight | 407.31 |
| Melting Point | 114.0~115.0℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Sitagliptin (CAS: 486460-32-6), as a new type of antidiabetic drug, Sitagliptin Phosphate has the advantages of glucose dependence, moderate hypoglycemic effect, increased secretion without hypoglycemia, effectively alleviated hunger, and had no side effects such as nausea, vomiting, edema and increased body weight. Sitagliptin phosphate as a single drug treatment in patients with type II diabetes can significantly reduce the level of glycosylated hemoglobin (HbA1c). It is a dipeptidyl peptidase-4 (DPP-4) inhibitor, which can improve the human body's ability to reduce high blood glucose level. Sitagliptin was developed by Merck & Co. and approved for medical use in the United States in 2006.