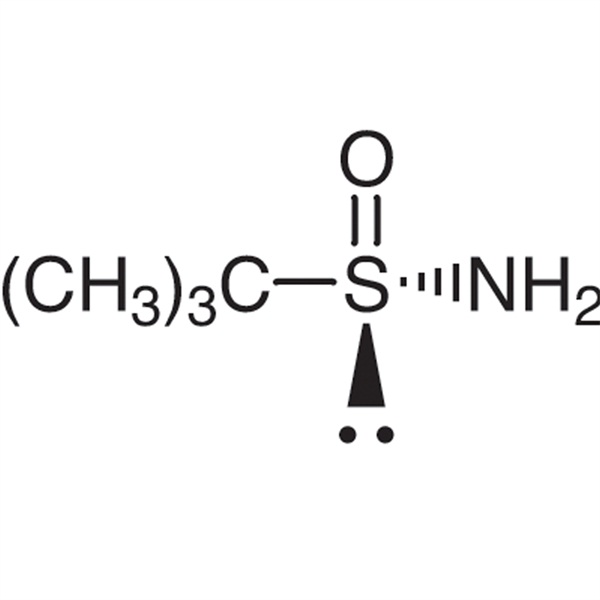
Chemical Properties:
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply with High Purity and Stable Quality (R)-(+)-tert-Butylsulfinamide CAS 196929-78-9 (S)-(-)-tert-Butylsulfinamide CAS 343338-28-3 Chiral Compounds, High Quality, Commercial Production| Item | Specifications |
| Appearance | White to Off-White Powder |
| Specific Rotation | -2.5° ~ -6.5° (C=1, CHCl3) |
| Purity (LC) | ≥99.0% |
| E.E (LC) | ≥99.0% |
| Total Impurities | ≤1.0% |
| Heavy Metals (as Pb) | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | Chiral Compounds; Pharmaceutical Intermediates |
Description:
Specifications:
Package & Storage:
| Chemical Name | (S)-(-)-tert-Butylsulfinamide |
| Synonyms | (S)-(-)-2-Methyl-2-Propanesulfinamide; S-BSN |
| CAS Number | 343338-28-3 |
| CAT Number | RF-CC219 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C4H11NOS |
| Molecular Weight | 121.2 |
| Melting Point | 97.0~101.0℃ |
| Solubility | Soluble in Methanol |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (S)-(-)-tert-Butylsulfinamide (CAS: 343338-28-3) with high quality, widely used in organic synthesis, synthesis of pharmaceutical intermediates and Active Pharmaceutical Ingredient (API) synthesis. Shanghai Ruifu Chemical Co., Ltd. plays an important role in the chiral chemistry, the company is committed to the production of chiral compounds. Our products are widely praised by customers. (S)-(-)-tert-Butylsulfinamide (CAS: 343338-28-3) is used in Suzuki reaction. It is also employed as a reagent for synthesizing chiral amines. It acts as a chiral auxiliary used in an asymmetric synthesis of trifluoroethylamines by conversion of trifluoroacetaldehyde to a chiral imine. It is also involved in the transformation of P,N-sulfinyl imine ligands through condensation with aldehydes and ketones, which can undergo iridium-catalyzed asymmetric hydrogenation of olefins. Further, it serves as a reagent for the preparation of chemicals and pharmaceutical intermediates.