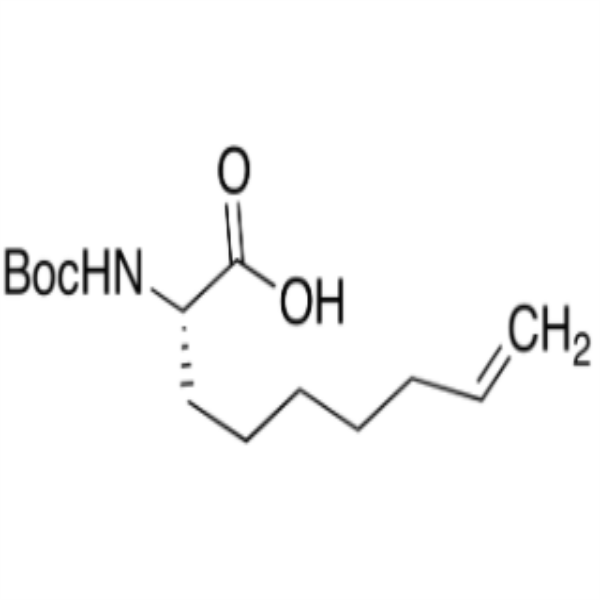
Chemical Properties:
Package: Fluorinated Bottle, 25kg/Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (S)-2-(Boc-Amino)non-8-Enoic Acid (CAS: 300831-21-4) with high quality, commercial production. Ruifu Chemical offers a wide range of chiral compounds. We can provide Certificate of Analysis (COA), worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | Brown Liquid |
| Purity / Analysis Method | >98.0% (GC) |
| e.e | >99.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediates of Paritaprevir (CAS: 1216941-48-8) |
Description:
Specifications:
Package & Storage:
| Chemical Name | (S)-2-(Boc-Amino)non-8-Enoic Acid |
| Synonyms | (S)-2-(tert-Butoxycarbonylamino)non-8-Enoic Acid; (S)-2-(Boc-Amino)-8-Nonenoic Acid; (2S)-2-[(2-Methylpropan-2-yl)oxycarbonylamino]non-8-Enoic Acid; (2S)-2-{[(tert-Butoxy)carbonyl]amino}non-8-Enoic Acid |
| CAS Number | 300831-21-4 |
| CAT Number | RF-CC347 |
| Stock Status | In Stock |
| Molecular Formula | C14H25NO4 |
| Molecular Weight | 271.35 |
| Density | 1.035±0.06 g/cm3 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
(S)-2-(Boc-Amino)non-8-Enoic Acid (CAS: 300831-21-4) can be used as an intermediate in the synthesis of Paritaprevir (CAS: 1216941-48-8) and related analogs. Paritaprevir is a second-generation NS3/4A protease inhibitor, is a component of the all-oral, interferon-free hepatitis C virus combination therapy developed by Enanta Pharmaceuticals and AbbVie. The fixed-dose tablet of Paritaprevir, Ombitasvir and Ritonavir taken in combination with Dasabuvir was approved for the treatment of chronic HCV genotype 1 in the USA and EU in 2014, and further approved for treatment of genotype 4 chronic HCV infection without cirrhosis by the US FDA in 2015.