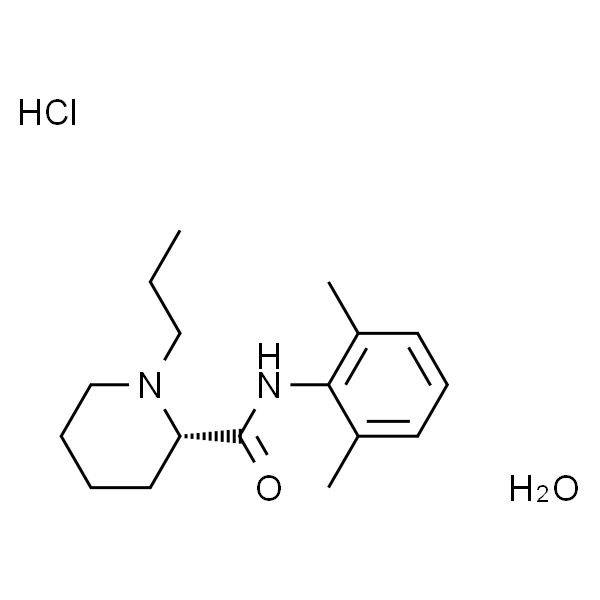
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer with High Purity and Stable Quality Chemical Name: Ropivacaine Hydrochloride Monohydrate Synonyms: Ropivacaine HCl.H2O CAS: 132112-35-7 An anaesthetic agent and blocks impulse conduction in nerve fibres through inhibiting sodium ion influx reversibly API USP Standard, High Quality, Commercial Production| Item | Specifications |
| Appearance | White Crystalline Powder |
| Identification | (1) It should be positive reaction (2) IR: Match with the reference standard |
| Color | The absorbance at 405nm is not more than 0.030 The absorbance at 436nm is not more than 0.025 |
| Solubility | Soluble in Water |
| pH | 4.5~6.0 |
| Clarity | Should be Clear |
| Water | 5.0%~6.0% |
| Specific Rotation | -210° ~ -255° (at 365 nm) |
| Heavy Metals | ≤10ppm |
| Limit of Ropivacaine Related Compound A | ≤10ppm |
| Related Compounds | |
| Bupivacaine | ≤0.20% |
| Other Individual Impurity | ≤0.10% |
| Total Impurity | ≤0.50% |
| Enantiomeric Purity | ≤0.50% |
| Residual Solvents | |
| Ethanol | ≤0.50% |
| Acetone | ≤0.50% |
| 4-Methyl-2-Pentanone | ≤0.50% |
| 2,6-Dimethylaniline | ≤10ppm |
| Assay | 98.5%~101.0% |
| Test Standard | United States Pharmacopeia (USP) Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Description:
Specifications:
Package & Storage:
| Chemical Name | Ropivacaine Hydrochloride Monohydrate |
| Synonyms | Ropivacaine HCl.H2O |
| CAS Number | 132112-35-7 |
| CAT Number | RF-API42 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C17H26N2O.ClH.H2O |
| Molecular Weight | 328.88 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Ropivacaine Hydrochloride Monohydrate (CAS: 132112-35-7) with high quality. Ropivacaine is a long-acting amide local anaesthetic agent and first produced as a pure enantiomer. It produces effects similar to other local anaesthetics via reversible inhibition of sodium ion influx in nerve fibres. Ropivacaine is less lipophilic than bupivacaine and is less likely to penetrate large myelinated motor fibres, resulting in a relatively reduced motor blockade. Thus, ropivacaine has a greater degree of motor sensory differentiation, which could be useful when motor blockade is undesirable. The reduced lipophilicity is also associated with decreased potential for central nervous system toxicity and cardiotoxicity.