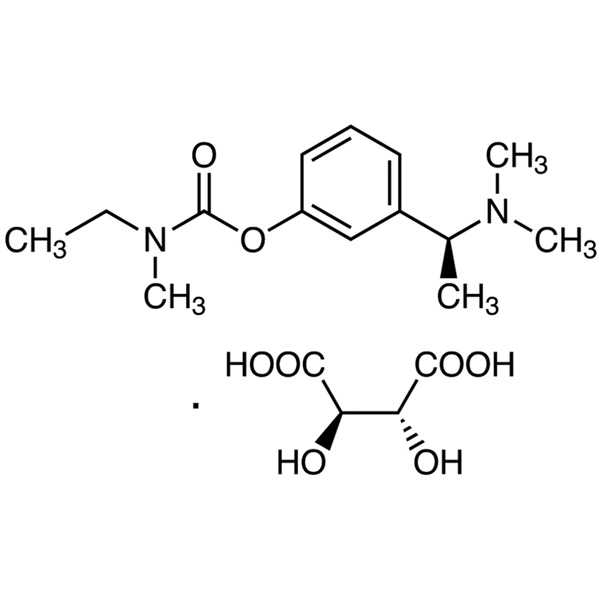
Chemical Properties:
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery. Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Rivastigmine Tartrate (CAS: 129101-54-8) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Rivastigmine Tartrate, Please contact: alvin@ruifuchem.comDescription:
Package/Storage/Shipping:
| Chemical Name | Rivastigmine Tartrate |
| Synonyms | Exelon; ENA-713; Rivastigmine L-Tartrate; Rivastigmine Hydrogen Tartrate; CS-118; S-Rivastigmine Tartrate; 3-[(S)-1-(Dimethylamino)ethyl]phenyl N-Ethyl-N-Methylcarbamate L-Tartrate; N-Ethyl-N-Methylcarbamic Acid 3-[(S)-1-(Dimethylamino)ethyl]phenyl Ester L-Tartrate |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 129101-54-8 |
| Related CAS | 123441-03-2 |
| Molecular Formula | C14H22N2O2·C4H6O6 |
| Molecular Weight | 400.43 g/mol |
| Melting Point | 124.0 to 128.0℃ |
| Specific Rotation [a]20/D | +4.0° to +7.0° (C=5, Methanol) |
| Solubility | Soluble in Methanol |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
Advantages:
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com 15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals. Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc. Advantages? Superior quality, affordable price, professional services and technical support, fast delivery. Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc. Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers. Factory Audit? Factory audit welcome. Please make an appointment in advance. MOQ? No MOQ. Small order is acceptable. Delivery Time? If within stock, three days delivery guaranteed. Transportation? By Express (FedEx, DHL), by Air, by Sea. Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided. Custom Synthesis? Can provide custom synthesis services to best fit your research needs. Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.FAQ:
Stable Supply: Maintain reasonable stock
Professional Service: One stop purchasing service
Technical Support: Technology solution available
Custom Synthesis Service: Ranged from grams to kilos
Fast Delivery: If within stock, three days delivery guaranteed
High Quality: Established a complete quality assurance system
Sufficient Capacity: Sufficient facilities and technicians
OEM Package: Custom package and label available
Specifications:
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Crystalline Powder | Complies |
| Assay | 98.0~102.0% (on the Anhydrous Basis) | 99.8% |
| Water by Karl Fischer | ≤0.50% | 0.15% |
| Residue on Ignition | ≤0.10% | 0.07% |
| Heavy Metals (Pb) | ≤20ppm | <10ppm |
| Phenol Impurity | ≤0.30% | <0.30% |
| DPTTA | ≤0.15% | <0.15% |
| Nor Impurity | ≤0.15% | <0.15% |
| Carbamate Impurity | ≤0.15% | <0.15% |
| Ether Impurity | ≤0.15% | <0.15% |
| Any Other Impurity | ≤0.10% | <0.10% |
| Total impurities | ≤0.50% | <0.50% |
| R-Enantiomer | ≤0.30% | <0.30% |
| Infrared Spectrum | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the USP35 Standard | |
129101-54-8 - Safety Information:
129101-54-8 - Application:
UN IDs UN 2811 6.1 / PGII WGK Germany 3 RTECS FA9550000 HS Code 29242990 Hazard Class 6.1 Packing Group IIIRivastigmine Tartrate (CAS: 129101-54-8) is the tartrate of rivastigmine, a drug for the treatment of Alzheimer's disease. Rivastigmine is a physostigmine derivative, which was successfully developed for the first time by Novartis, Switzerland. The trade name is exelon, and the molecule has a benzyl carbamate structure, it is a carbamate brain-selective cholinesterase inhibitor, which can inhibit acetylcholinesterase and butyrylcholinesterase at the same time, and promote cholinergic nerve conduction by delaying the degradation of acetylcholine released by cholinergic neurons. It can improve the cognitive dysfunction mediated by cholinergic, thereby improving the cognitive effect of patients with Alzheimer's disease. The plasma protein binding capacity of rivastigmine is weak, easy to pass through the blood-brain barrier, and has a high degree of brain selectivity. It can not only selectively act on the most vulnerable areas of the cerebral cortex and hippocampus, but also preferentially inhibit the dominant subtypes of AChE in the brain, which can reduce peripheral cholinergic side effects while producing curative effects. The half-life of rivastigmine tartrate in the body is short and the action time is long. Unlike tacrine, this product has a stronger inhibitory effect on G1 enzyme in the hippocampus and cortex. It is clinically used to treat mild to moderate Alzheimer's dementia, which is suspected of Alzheimer's disease or Alzheimer's disease.129101-54-8 - USP35 Standard:
Rivastigmine Tartrate C14H22N2O2·C4H6O6 400.42 Ethylmethylcarbamic acid, 3-[(S)-1-(dimethylamino)ethyl]phenyl ester, (2R,3R)-2,3-dihydroxybutanedioate; (S)-3-[1-(Dimethylamino)ethyl]phenyl ethylmethylcarbamate, hydrogen tartrate [129101-54-8]. Rivastigmine 250.34 [123441-03-2]. DEFINITION Rivastigmine Tartrate contains NLT 98.0% and NMT 102.0% of the labeled amount of C14H22N2O2·C4H6O6, calculated on the anhydrous basis. IDENTIFICATION • A. Infrared Absorption <197K> • B. The retention time of the major peak of the Sample solution corresponds to that of the System suitability solution, as obtained in the test for Organic Impurities, Procedure 2: Enantiomeric Purity. ASSAY • Procedure Buffer: 8.6 mg/mL of monobasic ammonium phosphate. Adjust with ammonia solution to a pH of 7.0. Mobile phase: Methanol, acetonitrile, and Buffer (15:15:70) System suitability solution: 0.05 mg/mL each of USP Rivastigmine Related Compound A RS and USP Rivastigmine Related Compound B RS in Mobile phase Standard solution: 0.2 mg/mL of USP Rivastigmine Tartrate RS in Mobile phase Sample solution: 0.2 mg/mL of Rivastigmine Tartrate in Mobile phase Chromatographic system (See Chromatography <621>, System Suitability.) Mode: LC Detector: UV 215 nm Column: 4.6-mm × 25-cm; 5-µm packing L7 Flow rate: 1.2 mL/min Injection size: 20 µL [Note-The flow rate may be adjusted to 1.5 mL/min, if needed, to achieve a recommended retention time of rivastigmine at about 10 min. ] System suitability Samples: System suitability solution and Standard solution Suitability requirements Resolution: NLT 1.5 between rivastigmine related compound A and rivastigmine related compound B, System suitability solution Column efficiency: NLT 5000 theoretical plates, Standard solution Tailing factor: NMT 3.0, Standard solution Relative standard deviation: NMT 2.0%, Standard solution Analysis Samples: Standard solution and Sample solution Calculate the percentage of C14H22N2O2·C4H6O6 in the portion of Rivastigmine Tartrate taken: Result = (rU/rS) × (CS/CU) × 100 rU = peak response from the Sample solution rS = peak response from the Standard solution CS = concentration of the Standard solution (mg/mL) CU = concentration of the Sample solution (mg/mL) Acceptance criteria: 98.0%-102.0% on the anhydrous basis IMPURITIES Inorganic Impurities • Residue on Ignition <281>: NMT 0.1% • Heavy Metals, Method II <231>: NMT 20 ppm Organic Impurities • Procedure 1 Mobile phase and System suitability solution: Proceed as directed in the Assay. Standard solution: 1.0 µg/mL of USP Rivastigmine Tartrate RS in Mobile phase Sample solution: 1.0 mg/mL of Rivastigmine Tartrate in Mobile phase Chromatographic system: Proceed as directed in the Assay. (See Chromatography <621>, System Suitability.) System suitability Samples: System suitability solution and Standard solution Suitability requirements Resolution: NLT 1.5 between rivastigmine related compound A and rivastigmine related compound B, System suitability solution Relative standard deviation: NMT 10%, Standard solution Analysis [Note-The run time is 8 times the retention time of the rivastigmine peak. ] Samples: Standard solution and Sample solution Calculate the percentage of any individual impurity in the portion of Rivastigmine Tartrate taken: Result = (rU/rS) × (CS/CU) × (1/F) × 100 rU = peak response for each impurity from the Sample solution rS = peak response from the Standard solution CS = concentration of USP Rivastigmine Tartrate RS in the Standard solution (mg/mL) CU = concentration of Rivastigmine Tartrate in the Sample solution (mg/mL) F = relative response factor (see Impurity Table 1) Acceptance criteria Individual impurities: See Impurity Table 1. Total impurities: NMT 0.5% Impurity Table 1| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria NMT % |
| Tartrate | 0.18 | — | Disregard |
| Phenol impuritya | 0.28 | 1.6 | 0.3 |
| DPTTAb | 0.46 | 0.83 | 0.15 |
| Nor impurityc | 0.57 | 1.2 | 0.15 |
| Rivastigmine | 1.0 | 1.0 | — |
| Carbamate impurityd | 4.1 | 1.3 | 0.15 |
| Ether impuritye | 6.5 | 1.4 | 0.15 |
| Any other impurity | — | 1.0 | 0.1 |
129101-54-8 - Precautions:
1. As an acetylcholinesterase inhibitor, rivastigmine bicartrate can improve the effect of succinylcholine muscle relaxant. Therefore, there should be a suitable intermittent period to stop taking this product before anesthesia. This product should be combined with other cholinergic or anticholinergic preparations, and caution should be taken (see [Drug Interaction]). 2. Due to their pharmacological effects, cholinesterase inhibitors may have vagus nerve tension effects on heart rate. As with other cholinergic drugs, caution must be exercised when it is given to patients with sick sinus syndrome or other heart block (see Adverse Reactions). 3. Cholinergic nerve excitement can cause increased gastric acid secretion. Although no evidence of significant deterioration of the corresponding symptoms was found during the clinical trial period, patients with a high risk of gastric ulcer, such as those with a history of ulcer disease or those receiving concomitant treatment with non-steroidal anti-inflammatory drugs, should be used with caution. 4. Like other cholinesterase inhibitors, patients with a history of asthma or other obstructive pulmonary disease should be used with caution.Rivastigmine Tartrate Intermediates: