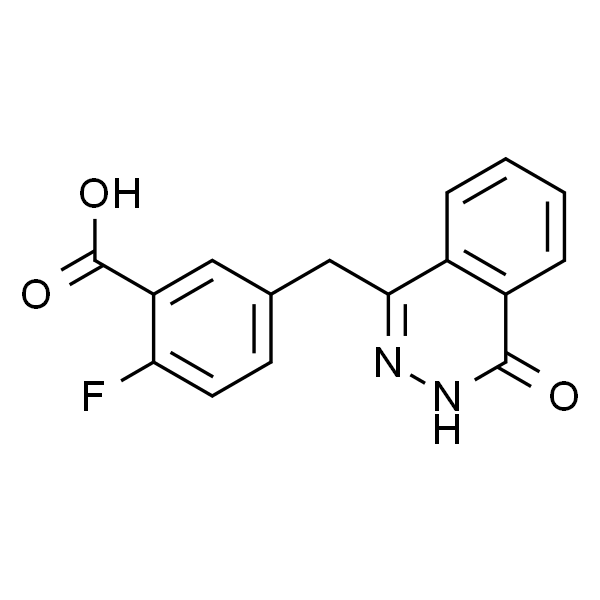
Chemical Properties:
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.High Purity, Commercial Production Olaparib and Related Intermediates: Olaparib CAS 763113-22-0 2-Fluoro-5-Formylbenzonitrile CAS 218301-22-5 2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid CAS 763114-26-7 1-(Cyclopropylcarbonyl)piperazine Hydrochloride CAS 1021298-67-8 3-Oxo-1,3-Dihydroisobenzofuran-1-Ylphosphonic Acid CAS 61260-15-9| Item | Specifications |
| Appearance | Yellow to Off-White Powder |
| Assay | ≥98.5% |
| Moisture (K.F) | ≤0.50% |
| Total Impurities | ≤1.5% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Olaparib (CAS: 763113-22-0) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid |
| Synonyms | 2-Fluoro-5-[(4-oxo-3H-phthalazin-1-yl)methyl]benzoic acid |
| CAS Number | 763114-26-7 |
| CAT Number | RF-PI450 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C16H11FN2O3 |
| Molecular Weight | 298.27 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (CAS: 763114-26-7) is an intermediate used in the synthesis of Olaparib (CAS: 763113-22-0). Olaparib, marketed by AstraZeneca under the brand name Lynparza, was approved in the USA in December 2014 as a targeted, single-agent therapy for the treatment of germline BRCA-mediated advanced ovarian cancer.Olaparib, originally developed by KuDOS pharmaceuticals and later by AstraZeneca, functions as a poly ADP ribose polymerase inhibitor and has been specifically approved for patients who have received three or more treatments of chemotherapy. In clinical trials, the drug prolonged progression- free survival for patients suffering from platinum-sensitive recurrent serous ovarian cancer. Olaparib is also currently in various phases of investigation for treatment of breast, gastric, prostate, pancreatic and non-small cell lung cancer.