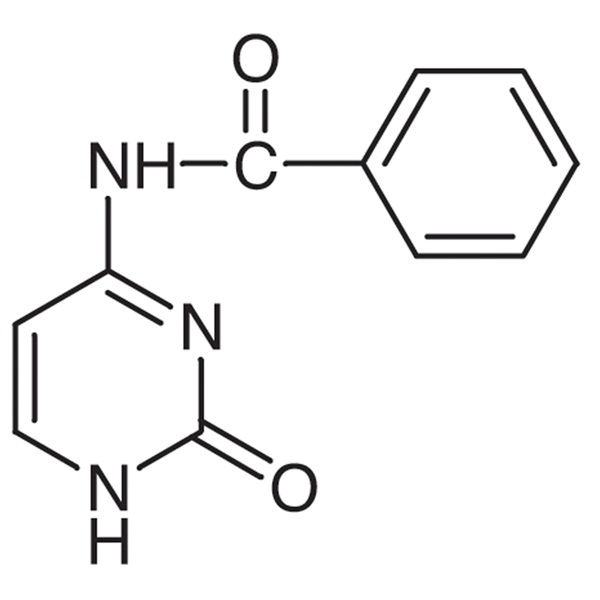
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Commercial Supply Sofosbuvir and Related Intermediates: Sofosbuvir CAS: 1190307-88-0 N4-Benzoylcytosine CAS: 26661-13-2 triphenyl(1-[ethoxycarbonyl]ethylidene)phosphorane CAS: 21382-82-1 Pentafluorophenol CAS: 771-61-9 N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-L-alanine 1-Methylethyl ester CAS: 1334513-02-8| Item | Specifications |
| Appearance | White or Off-White Powder |
| Identification | HPLC |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Moisture (K.F) | ≤0.50% |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Cytosine | ≤0.50% |
| Heavy Metals | ≤20ppm |
| Test Standard | Enterprise Standard |
| Upstream Product | Cytosine CAS: 71-30-7 |
| Usage | Intermediate of Sofosbuvir (CAS: 1190307-88-0) |
Description:
Specifications:
Package & Storage:
| Name | N4-Benzoylcytosine |
| CAS Number | 26661-13-2 |
| CAT Number | RF-PI178 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H9N3O2 |
| Molecular Weight | 215.21 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Manufacturer with High Purity and Stable Quality Commercial Supply Sofosbuvir (CAS: 1190307-88-0) and Related Intermediates: Sofosbuvir CAS: 1190307-88-0 N4-Benzoylcytosine CAS: 26661-13-2 triphenyl(1-[ethoxycarbonyl]ethylidene)phosphorane CAS: 21382-82-1 Pentafluorophenol CAS: 771-61-9 N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-L-alanine 1-Methylethyl ester CAS: 1334513-02-8 Sofosbuvir (CAS: 1190307-88-0) is a drug used for the treatment of hepatitis C. It is recommended to be used in combination with other drugs (such as velpatasvir) for the first-line treatment for HCV genotypes 1, 2, 3, 4, 5, and 6. It takes effect through acting as a nucleotide analog inhibitor, being capable of specially inhibiting the HCV NS5B (non-structural protein 5B) RNA-dependent RNA polymerase. Oral sofosbuvir was generally well tolerated in patients with chronic hepatitis C.