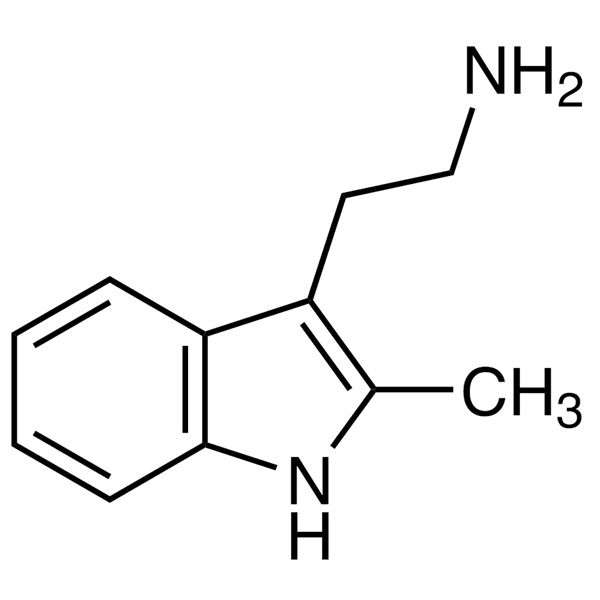
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply With High Quality, Commercial Production Chemical Name: 2-Methyltryptamine CAS: 2731-06-8| Item | Specifications |
| Appearance | Brown Solid |
| Purity / Analysis Method | >99.0% (HPLC Area) |
| 1-HNMR | Consistent With Structure |
| Loss on Drying | <1.00% |
| Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Heavy Metals (as Pb) | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates; Panobinostat Intermediate |
Description:
Specifications:
Package & Storage:
| Chemical Name | 2-Methyltryptamine |
| Synonyms | 2-Methylindole-3-Ethylamine; 2-(2-Methyl-1H-indol-3-yl)ethanamine; 3-(2-Aminoethyl)-2-Methylindole |
| CAS Number | 2731-06-8 |
| CAT Number | RF-PI1480 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H14N2 |
| Molecular Weight | 174.25 |
| Melting Point | 106.0 to 110.0℃ |
| Boiling Point | 177℃/1.5mmHg(lit.) |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2-Methyltryptamine (CAS: 2731-06-8) is a key starting material in the synthesis of Panobinostat (LBH589) (CAS: 404950-80-7). Panobinostat is a potent inhibitor of all histone deacetylases (HDACs), with Ki values ranging from 0.6 to 31 nM for HDAC1-11. Through this action, it leads to acetylation of a range of cellular proteins, resulting in cell cycle arrest and apoptosis in cancer cells. Panobinostat is the first HDAC inhibitor was approved by FDA for the treatment of multiple myeloma. Panobinostat is marketed as Farydak, is used in combination with Bortezomib (CAS: 179324-69-7) and Dexamethasone (CAS: 50-02-2) to treat relapsed multiple myeloma after prior treatment with Bortezomib and an immunomodulator.