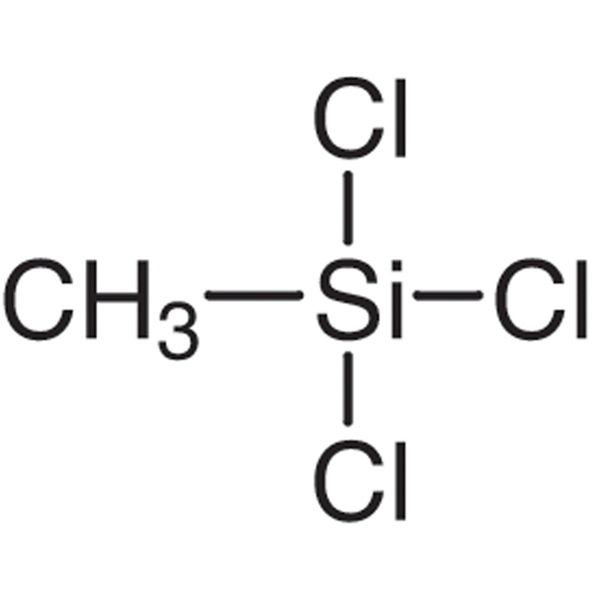
Chemical Properties:
Package: Fluorinated Bottle, 25kg/Drum, 170kg/Drum or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Methyltrichlorosilane (CAS: 75-79-6) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | Colorless Liquid |
| Purity / Analysis Method | >99.0% (GC) |
| Refractive Index n20/D | 1.4090~1.4120 |
| Specific Gravity (20/20℃) | 1.2770~1.2810 |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Silicon Compounds; Silane Coupling Agents |
Description:
Specifications:
Package & Storage:
| Chemical Name | Methyltrichlorosilane |
| Synonyms | Trichloro(methyl)silane; Trichloromethylsilane; MTS |
| CAS Number | 75-79-6 |
| CAT Number | RF-PI2133 |
| Stock Status | In Stock, Production Capacity 100 Tons/Month |
| Molecular Formula | CH3SiCl3 |
| Molecular Weight | 149.48 |
| Sensitive | Moisture Sensitive, Light Sensitive |
| Melting Point | -90℃(lit.) |
| Boiling Point | 66℃ |
| Water Solubility | Reacts With Water |
| Storage Temp. | Flammables Area |
| Hydrolytic Sensitivity | 8: Reacts Rapidly with Moisture, Water, Protic Solvents |
| Hazard | Fuming Liquid with a Pungent Odor. Vapor and Liquid may Cause Burns. Denser than Water. Vapors are Heavier than Air. Flammable, Dangerous Fire Risk, may Formexplosive Mixture with Air. Strong Irritant. |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Methyltrichlorosilane, also known as Trichloro(methyl)silane, (CAS: 75-79-6), Silicon Compounds, Silane Coupling Agents, Self-Assembled Monolayer Forming Agents. Methyltrichlorosilane is used in production of methyl silicone resins, its vapor reacts with water on surfaces to give a thin layer of methylpolysiloxane which make it a water-repellent film. A combination of methyltrichlorosilane and sodium iodide can be used to cleave carbon-oxygen bonds such as methyl ethers. It is used as a precursor for forming various cross-linked siloxane polymers. Trichloromethylsilane is the starting material for the production of pure silicon for manufacture of semiconductors and optical fibers. Intermediate for silicones. Methyltrichlorosilane is widely used as precursor to organosilicon compounds; silylating agent and Lewis acid. MeSiCl3 is an effective Lewis acid in the condensation of carboxylic acids with alcohols and amines. React with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. Health Hazard: As with other chlorosilanes, acute exposures may be highly toxic and may cause death or permanent injury after very short exposures to small quantitites. Chronic exposures may be moderately toxic and involve irreversible and reversible changes. Skin contact may produce severe burns with pain and risk of secondary infections. Ingestion may produce oral, esophageal, and stomach burns, intensity will vary from mild to very severe, gastrointestinal damage is rare but may occur. Fire Hazard: Toxic hydrogen chloride and phosgene gases may form in fires. Reacts with water or steam to form hydrochloric acid. Vapor forms flammable mixture with air. May form explosive mixture in air. Avoid contact with water or moist air.