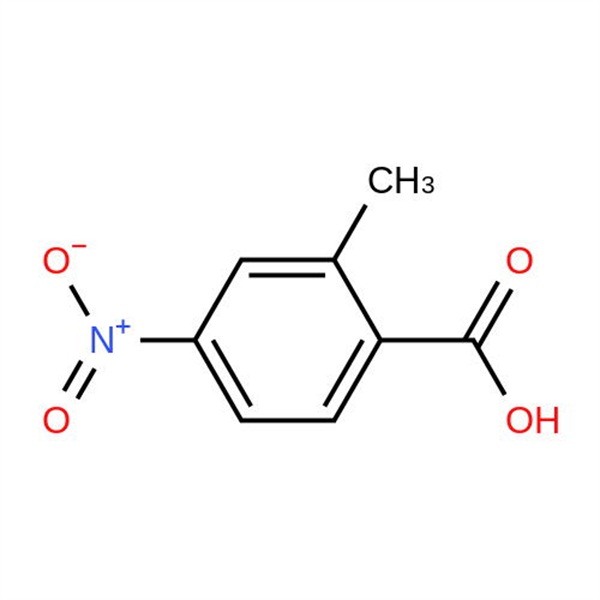
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply, High Quality, Commercial Production Tolvaptan and Related Intermediates: Tolvaptan CAS 150683-30-0 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one CAS 160129-45-3 o-Toluoyl Chloride CAS 933-88-0 4-Amino-2-Methylbenzoic Acid CAS 2486-75-1 2-Methyl-4-Nitrobenzoic Acid CAS 1975-51-5| Item | Specifications |
| Appearance | Almost White or Pale Yellow Crystal Powder |
| 1H-NMR | Consistent with Structure |
| Purity / Analysis Method | ≥98.0% (HPLC) |
| Melting Point | 153.0~156.0℃ |
| Moisture (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Total Impurities | ≤2.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Tolvaptan (CAS 150683-30-0) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 2-Methyl-4-Nitrobenzoic Acid |
| Synonyms | 4-Nitro-o-Toluic Acid; 4-Nitro-2-Methylbenzoic Acid |
| CAS Number | 1975-51-5 |
| CAT Number | RF-PI397 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H7NO4 |
| Molecular Weight | 181.15 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2-Methyl-4-Nitrobenzoic Acid (CAS 1975-51-5) is used as a pharmaceutical intermediate in the synthesis of Tolvaptan (CAS 150683-30-0). Tolvaptan is a selective, competitive arginine vasopressin receptor 2 antagonist with an IC50 of 1.28μM for the inhibition of AVP-induced platelet aggregation. Tolvaptan is used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan was approved by the U.S. Food and Drug Administration on May 19, 2009, and is sold by Otsuka Pharmaceutical Co. under the trade name Samsca.