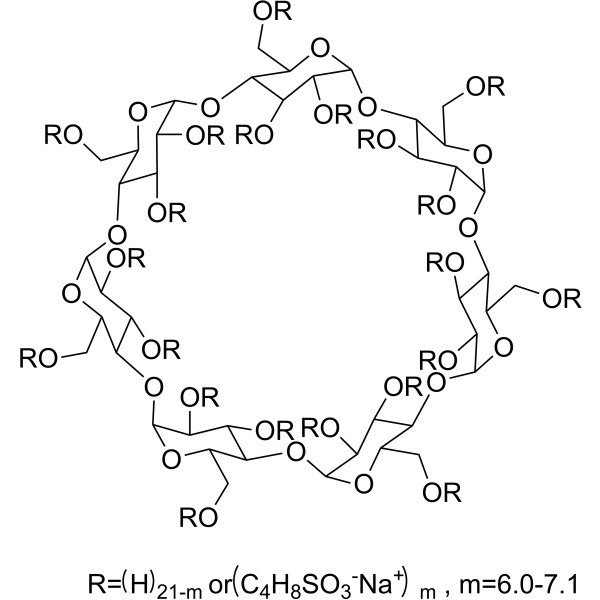
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Preserve in well-closed containers and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery. Ruifu Chemical is the leading manufacturer of Betadex Sulfobutyl Ether Sodium (SBE-β-CD; Captisol) (CAS: 182410-00-0) with high quality. Ruifu can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Betadex Sulfobutyl Ether Sodium, Please contact: alvin@ruifuchem.comDescription:
Package/Storage/Shipping:
| Chemical Name | Betadex Sulfobutyl Ether Sodium |
| Synonyms | SBE-β-CD; SBE-beta-CD; Captisol; Sodium Sulfobutylether β-Cyclodextrin; Sodium Sulphobutylether-beta-Cyclodextrin; Sulfobutylether beta-Cyclodextrin; Beta-Cyclodextrin Sulfobutyl Ethers Sodium Salts; β-Cyclodextrin Sulfobutyl Ethers Sodium Salts |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 182410-00-0 |
| Molecular Formula | C42H70O35•xNa•x(C4H9O3S) |
| Molecular Weight | (1134.99).x(22.99).x(137.17) g/mol |
| Melting Point | 202.0~204.0℃(dec.) |
| Solubility | Soluble in Water. Insoluble in Acetone, Methanol, Chloroform |
| HS Code | 3505100000 |
| COA & MSDS | Available |
| Sample | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
Advantages:
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com 15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals. Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc. Advantages? Superior quality, affordable price, professional services and technical support, fast delivery. Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc. Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers. Factory Audit? Factory audit welcome. Please make an appointment in advance. MOQ? No MOQ. Small order is acceptable. Delivery Time? If within stock, three days delivery guaranteed. Transportation? By Express (FedEx, DHL), by Air, by Sea. Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided. Custom Synthesis? Can provide custom synthesis services to best fit your research needs. Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc. FAQ:
Stable Supply: Maintain reasonable stock
Professional Service: One stop purchasing service
Technical Support: Technology solution available
Custom Synthesis Service: Ranged from grams to kilos
Fast Delivery: If within stock, three days delivery guaranteed
High Quality: Established a complete quality assurance system
Sufficient Capacity: Sufficient facilities and technicians
OEM Package: Custom package and label available
Specifications:
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Amorphous Powder | Conform |
| Identification IR | Same absorption bands as USP Betadex Sulfobutyl Ether Sodium RS | Conform |
| Identification HPLC | The retention time of the major peak of sample solution corresponds to the standard solution | Conform |
| Average Degree of Substitution | Conform | Conform |
| Identification Sodium | Identify test are positive for Sodium | Conform |
| Assay | 95.0%~105.0% | 99.49% |
| Beta Cyclodextrin | ≤0.10% | Not Detected |
| 1,4-Butane Sultone | ≤0.5ppm | 0.19ppm |
| Sodium Chloride | ≤0.20% | 0.003% |
| 4-Hydroxybutane-1-Sulfonic Acid | ≤0.09% | Not Detected |
| Bis(4-Sulfobtyl) Ether Disodium | ≤0.05% | Not Detected |
| Bacterial Endotoxins | ≤20EU/g | <5EU/g |
| The Total Aerobic Microbial Count | ≤100cfu/g | <10cfu/g |
| The Total Combined Moulds and Yeasts Count | ≤50cfu/g | <10cfu/g |
| Escherichia Coli | Absence | Not Detected |
| Clarity of Solution | 30%(w/v) solution is clear and essentially free from particles of foreign matter. | Conform |
| Average Degree of Substitution | 6.2~6.9 | 6.5 |
| Peak I | 0.0~0.3 | 0 |
| Peak II | 0.0~0.9 | 0.62 |
| Peak III | 0.5~5.0 | 1.41 |
| Peak IV | 2.0~10.0 | 4.46 |
| Peak V | 10.0~20.0 | 11.72 |
| Peak VI | 15.0~25.0 | 20.75 |
| Peak VII | 20.0~30.0 | 29.04 |
| Peak VIII | 10.0~25.0 | 21.59 |
| PeakI X | 2.0~12.0 | 7.83 |
| Peak X | 0.0~4.0 | 2.57 |
| pH | 4.0~6.8 | 4.8 |
| Water Content | ≤10.0% | 4.9% |
| Infrared Spectrum | Conforms to Structure | Complies |
| Conclusion | This product by inspection accords with the standard USP35 |
182410-00-0 - Application:
Betadex Sulfobutyl Ether Sodium (SBE-β-CD; Captisol) (CAS: 182410-00-0) is a new type of chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs. Betadex Sulfobutyl Ether Sodium is a new type of pharmaceutical preparation excipient, belonging to a sulfonic acid derivative of anionic highly water-soluble cyclodextrin. It can be well combined with drug molecules to form non-covalent complexes, which improves the stability, water solubility, and safety of the drug, and effectively improves the biological activity of the drug molecule. Its nephrotoxicity is small, and it can alleviate drug hemolysis., Control the rate of drug release. Betadex Sulfobutyl Ether Sodium can form noncovalent complexes with many types of compounds including small organic molecules, peptides, and proteins. It can also enhance their solubility and stability in water. The first application of sulfobutylether bcyclodextrin was in injectable preparations; it can also be used in oral solid and liquid dosage forms, and ophthalmic, inhalation, and intranasal formulations. Betadex Sulfobutyl Ether Sodium can function as an osmotic agent and/or a solubilizer for controlled-release delivery, and has antimicrobial preservative properties when present at sufficient concentrations. The amount of Betadex Sulfobutyl Ether Sodium that may be used is dependent on the purpose for inclusion in the formulation, the route of administration, and the ability of the cyclodextrin to complex with the drug being delivered.182410-00-0 - USP35 Standard:
Betadex Sulfobutyl Ether Sodium C42H70−nO35 · (C4H8SO3Na)n 2163 when n = 6.5 Beta cyclodextrin sulfobutyl ethers, sodium salts; Beta cyclodextrin sulfobutyl ether sodium [182410-00-0]. DEFINITION Betadex Sulfobutyl Ether Sodium is prepared by alkylation of betadex using 1,4-butane sultone under basic conditions The average degree of substitution in betadex is NLT 6.2 and NMT 6.9. It contains NLT 95.0% and NMT 105.0% of C42H70−nO35 · (C4H8SO3Na)n (n = 6.2–6.9), calculated on the anhydrous basis. IDENTIFICATION • A. INFRARED ABSORPTION <197K> • B. The retention time of the major peak of the Sample solution corresponds to that of the standard solution, as obtained in the Assay. • C. It meets the requirements of the test for Average Degree of Substitution. • D. IDENTIFICATION TESTS-GENERAL, Sodium 〈191〉 ASSAY • PROCEDURE Mobile phase: 0.1 M potassium nitrate in a mixture of acetonitrile and water (1:4) Standard solution: 10 mg/mL of USP Betadex Sulfobutyl Ether Sodium RS in Mobile phase Sample solution: 10 mg/mL of Betadex Sulfobutyl Ether Sodium in Mobile phase Chromatographic system (See Chromatography <621>, System Suitability.) Mode: LC Detector: Refractive index Detector temperature: 35 ± 2° Column: 7.8-mm × 30-cm analytical column; packing L37. [NOTE-Rinse the column with a solution of acetonitrile and water (1:9) at the completion of the run series.] Flow rate: 1.0 mL/min Injection size: 20 µL System suitability . Sample: Standard solution Suitability requirements Relative standard deviation: NMT 2.0% Analysis Samples: Standard solution and Sample solution Calculate the percentage of betadex sulfobutyl ether sodium [C42H70−nO35 · (C4H8SO3Na)n] in the portion of Betadex Sulfobutyl Ether Sodium taken: Result = (rU/rS) × (CS/CU) × 100 rU = peak response for betadex sulfobutyl ether sodium from the Sample solution rS = peak response for betadex sulfobutyl ether sodium from the Standard solution CS = concentration of USP Betadex Sulfobutyl Ether Sodium RS in the Standard solution (mg/mL) CU = concentration of Betadex Sulfobutyl Ether Sodium in the Sample solution (mg/mL) Acceptance criteria: 95.0%~105.0% on the anhydrous basis IMPURITIES • HEAVY METALS, Method II <231>: NMT 5 ppm • LIMIT OF BETA CYCLODEXTRIN (BETADEX) Solution A: 25 mM sodium hydroxide Solution B: 250 mM sodium hydroxide and 1 M potassium nitrate Mobile phase: See Table 1. Table 1 | Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 4 | 100 | 0 |
| 5 | 0 | 100 |
| 10 | 0 | 100 |
| 11 | 100 | 0 |
| 20 | 100 | 0 |
Standard solution: 2 µg/mL of USP Beta Cyclodextrin RS Sample solution: 2 mg/mL of Betadex Sulfobutyl Ether Sodium Chromatographic system (See Chromatography <621>, System Suitability and Ion Chromatography <1065>.) Mode: IC Detector: Pulsed amperometry (amperometric cell with gold working electrode and silver reference electrode) Column Guard: 4.0-mm × 5-cm anion-exchange; packing L61 Analytical: 4.0-mm × 25-cm anion-exchange; packing L61 Column temperature: 50 ± 2° Flow rate: 1.0 mL/min Injection size: 20 µL Waveform for pulsed amperometric detector: See Table 2. Table 2 | Time (s) | Voltage (V) |
| 0.00 | 0.10 |
| 0.30 | Start integration |
| 0.50 | 0.10 |
| 0.50 | Stop integration |
| 0.51 | 0.60 |
| 0.60 | -0.60 |
| 0.65 | -0.60 |
System suitability Sample: Standard solution Suitability requirements Relative standard deviation: NMT 5.0% Analysis Samples: Standard solution and Sample solution Calculate the percentage of beta cyclodextrin (betadex) in the portion of Betadex Sulfobutyl Ether Sodium taken: Result = (rU/rS) × (CS/CU) × F × 100 rU = peak response for beta cyclodextrin from the Sample solution rS = peak response for beta cyclodextrin from the Standard solution CS = concentration of USP Beta Cyclodextrin RS in the Standard solution (µg/mL) CU = concentration of Betadex Sulfobutyl Ether Sodium in the Sample solution (mg/mL) F = conversion factor (10-3 mg/µg) Acceptance criteria: NMT 0.1% • LIMIT OF 1,4-BUTANE SULTONE Internal standard solution: 0.25 µg/mL of diethyl sulfone Standard stock solution A: 0.5 µg/mL of 1,4-butane sultone Standard stock solution B: 1.0 µg/mL of 1,4-butane sultone Standard stock solution C: 2.0 µg/mL of 1,4-butane sultone Sample stock solution: 250 mg/mL of Betadex Sulfobutyl Ether Sodium in the Internal standard solution Blank solution, and Sample solutions A, B, C, and D: Follow Table 3 to place the quantities of Internal standard solution, each Standard stock solution, Sample stock solution, water, or methylene chloride in each glass test tube with a stopper. [NOTE-A screw-capped, 10-mL test tube is suitable.] Mix on a vortex mixer each test tube for 30 s, and allow it stand for at least 5 min or until complete separation of the phase. Extract the organic phase into a GC vial and seal. [NOTE-With great care take the minimum possible amount of aqueous phase.] Added quantities of 1,4-butane sultone in Sample solutions A, B, C, and D are 0.5, 1.0, 2.0, and 0 µg, respectively. Table 3 | Sample Name | Solution 1 Added (mL) | Solution 2 Added (mL) | Methylene Chloride Added (mL) |
| Blank solution | Internal standard solution, 4.0 | Water, 1.0 | 1.0 |
| Sample solution A | Sample stock solution, 4.0 | Standard stock solution A, 1.0 | 1.0 |
| Sample solution B | Sample stock solution, 4.0 | Standard stock solution B, 1.0 | 1.0 |
| Sample solution C | Sample stock solution, 4.0 | Standard stock solution C, 1.0 | 1.0 |
| Sample solution D | Sample stock solution, 4.0 | Water, 1.0 | 1.0 |
[NOTE-Prepare immediately before use.] Chromatographic system (See Chromatography <621>, System Suitability.) Mode: GC Detector: Flame ionization Column: 0.32-mm × 25-m fused-silica capillary column; 0.5-µm layer of phase G46 Temperature Detector: 270° Injection port: 200° Column: See the temperature program in Table 4 Table 4 Initial Temperature (°) Temperature Ramp (°/min) Final Temperature (°) Hold Time at Final Temperature (min) 100 10 200 - 200 35 270 5 Carrier gas: Helium, typically at 12 psi inlet pressure Injection size: 1.0 µL Injection type: Splitless injection for 0.5 min, then split at 50 mL/min. [NOTE-The use of an appropriate splitless injection liner is recommended.] System suitability Sample: Sample solution B [NOTE-The relative retention times for diethyl sulfone and 1,4-butane sultone are 0.7 and 1.0, respectively.] Suitability requirements Relative standard deviation: NMT 10.0% Analysis Samples: Blank solution, Sample solutions A, B, C, and D Correct the ratio of peak responses of the 1,4-butane sultone to diethyl sulfone in Sample solution A, B, C, or D by subtracting the ratio of peak responses of the 1,4-butane sultone to ethyl sulfone in the Blank solution. Plot the corrected ratio of peak response of 1,4-butane sultone to peak response of diethyl sulfone in Sample solution A, B, C or D, versus the added quantity, in µg, of 1,4-butane sultone. Extrapolate the line joining the points on the graph until it meets the quantity axis. The distance between this point and the intersection of the axes represents the quantity of 1,4-butane sultone, A, in µg, in the 4-mL portion of Sample stock solution. Calculate the content of 1,4-butane sultone in the portion of Betadex Sulfobutyl Ether Sodium taken: Result = A/(VExt × CU × F) A = determined above VExt = volume of the Sample stock solution used in the extraction step, 4.0 mL CU = concentration of Betadex Sulfobutyl Ether Sodium in the Sample stock solution (mg/mL) F = conversion factor (10-3 g/mg) Acceptance criteria: hydroxybutane-1-sulfonic acid, or NMT 0.5 ppm • LIMIT OF SODIUM CHLORIDE, 4-HYDROXYBUTANE-1-SULFONIC ACID, AND BIS(4-SULFOBUTYL) ETHER DISODIUM Solution A: 5 mM sodium hydroxide, degas in a closed vessel for 15 min Solution B: 25 mM sodium hydroxide, degas in a closed vessel for 15 min Mobile phase: See Table 5 Table 5 | Time (min) | Solution A (%) | Solution B(%) |
| 0 | 100 | 0 |
| 4 | 100 | 0 |
| 10 | 70 | 30 |
| 24 | 70 | 30 |
| 25 | 100 | 0 |
| 40 | 100 | 0 |
Column wash solution A: 50 mM sodium citrate Column wash solution B: 150 mM sodium hydroxide Standard solution: Prepare a solution having known concentrations of 8 µg/mL of USP Sodium Chloride RS, 4µg/mL of 4-hydroxybutane-1-sulfonic acid, and 4 µg/mL of bis(4-sulfobutyl) ether disodium. Sample solution: 4 mg/mL of Betadex Sulfobutyl Ether Sodium Chromatographic system (See Chromatography <621>, System Suitability and Ion Chromatography <1065>.) Mode: IC Detector: Conductivity Range: 30 µS Current: 100 mA Column: [NOTE-At the end of each run, clean the column using Column wash solution A at a flow rate of 1 mL/min for 35 min then using Column wash solution B at the same flow rate for 35 min.] Guard: 4.0-mm × 5.0-cm anion-exchange; packing L61 Analytical: 4.0-mm × 25-cm anion-exchange; packing L61 Column temperature: 30° Suppressor: Micromembrane anion autosuppressor1 or a suitable chemical suppression system Suppressant: Autosuppression Flow rate: 1.0 mL/min Injection size: 20 µL System suitability Sample: Standard solution [NOTE-Relative retention times are provided for information only. The relative retention times for 4-hydroxybutane-1-sulfonate ion, chloride ion, and bis(sulfobutyl) ether ion are 1.0, 1.4, and 8.6, respectively.] Suitability requirements Resolution: NLT 2.0 Relative standard deviation: NMT 10.0% Analysis Samples: Standard solution and Sample solution Calculate the percentage of sodium chloride, 4-hydroxybutane-1-sulfonic acid, or bis(sulfobutyl) ether disodium in the portion of Betadex Sulfobutyl Ether Sodium taken: Result = (rU/rS) × (CS/CU) × F × 100 rU = peak response for sodium chloride, 4-hydroxybutane-1-sulfonic acid, or bis(sulfobutyl) ether disodium from the Sample solution rS = peak response for sodium chloride, 4-hydroxybutane-1-sulfonic acid, or bis(sulfobutyl) ether disodium from the Standard solution CS = concentration of sodium chloride, 4-hydroxybutane-1-sulfonic acid, or bis(sulfobutyl) ether disodium in the Standard solution (µg/mL) CU = concentration of Betadex Sulfobutyl Ether Sodium in the Sample solution (mg/mL) F = conversion factor (10−3 0 100 0 mg/µg) Acceptance criteria Sodium chloride: NMT 0.2% 4-Hydroxybutane-1-sulfonic acid: NMT 0.09% Bis(sulfobutyl) ether disodium: NMT 0.05% SPECIFIC TESTS BACTERIAL ENDOTOXINS TEST <85>: The level of bacteria endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Betadex Sulfobutyl Ether Sodium is used can be met. Where the label states that Betadex Sulfobutyl Ether Sodium must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Betadex Sulfobutyl Ether Sodium is used can be met. • MICROBIAL ENUMERATION TESTS <61> and TESTS FOR SPECIFIED MICROORGANISMS <62>: The total aerobic microbial count does not exceed 100 cfu/g, and the total combined molds and yeasts count does not exceed 50 cfu/g. It meets the requirements of the test for absence of Escherichia coli. • CLARITY OF SOLUTION Sample solution: 30% (w/v) solution Analysis: Examine the Sample solution using a light box against white and black backgrounds, and record the presence of any haze, fluorescence, fibers, specks, or other foreign matter. Acceptance criteria: The solution is clear, and essentially free from particles of foreign matter. • AVERAGE DEGREE OF SUBSTITUTION Run electrolyte: 30 mM benzoic acid and adjusted to a pH that is suitable for the instrument used by addition of 100 mM tris(hydroxymethyl) aminomethane buffer. [NOTE-Due to variation between capillaries, a single universally applicable electrolyte pH is not specified. Instead, the optimal pH associated with each individual capillary should be determined according to the instrumental manual.] Standard solution: 10 mg/mL of USP Betadex Sulfobutyl Ether Sodium RS Sample solution: 10 mg/mL of Betadex Sulfobutyl Ether Sodium Capillary rinsing procedure: Use separate run electrolyte vials for capillary rinse and sample analysis. Perform pre-analysis rinses on a daily basis before each analysis: rinse the capillary with 0.1 N sodium hydroxide for 30 min, with water for NLT 2 h, and with Run electrolyte for NLT 1 h. Perform pre-injection rinses prior to each injection as follows. Rinse the capillary with 0.1 N sodium hydroxide for NLT 1 min, and with Run electrolyte for NLT 3 min. If a new capillary is being used, in addition to the regular rinses described above, a new capillary requires rinsing before its first use. Rinse the new capillary with 1 M sodium hydroxide for 1 h, followed by a 2-h water rinse. Electrophoretic system (See Capillary Electrophoresis <1053>.) Mode: High-performance CE Detector: Inverse UV 200 nm, with a bandwidth of 20nm. [NOTE-A detection wavelength of 205 nm with a bandwidth of 10 nm may be used as an alternative.] Column: Sodium Peaks I–X (% Peak Area) 50-µm × 50-cm fused silica column Column temperature: 25° Applied voltage: 0.00 to +30.00 kV linear ramp over 10 min, then at 30 kV for a further 20 min Injection size: Equal volumes at 0.5 psi for 10 s System suitability Sample: Standard solution [NOTE-See Table 6 for the approximate relative migration times for betadex sulfobutyl ether sodium peaks I–X (betadex sulfobutyl ether sodium peaks I, II, III, ..., X, contains beta cyclodextrin molecule with 1, 2, 3, ..., 10 sulfobutyl substituent(s), respectively). The relative migration times are for informational purposes only to aid in peak identification.] Table 6 | Betadex Sulfobutyl Ether Sodium Peaks I–X | Relative Migration Time |
| I | 0.58 |
| II | 0.63 |
| III | 0.69 |
| IV | 0.77 |
| V | 0.83 |
| VI | 0.91 |
| VII | 1.00 |
| VIII | 1.10 |
| IX | 1.20 |
| X | 1.30 |
Suitability requirements Resolution: NLT 0.9, between betadex sulfobuty ether sodium peak IX and betadex sulfobutyl ether sodium peak X Analysis Samples: Run electrolyte, water, Standard solution, and Sample solution Inject the Standard solution and Sample solution by applying differential pressure of 0.5 psi, equivalent to 34 mbar, for 10 s, followed by injection of Run electrolyte at 0.5 psi for 2 s. [NOTE-Pressure injections should be made with a vial of water or Run electrolyte at the outlet end of the capillary.] Record the electropherograms, and measure the peak responses for the individual betadex sulfobutyl ether sodium peaks (I to X). Calculate the corrected peak area, AI, for each peak in the eletropherogram: Corrected Peak Area A = Peak Area x Effective Capillary Length (cm) / Migration Time Normalize the corrected peak areas by presenting each as a percentage of the total corrected substitution envelope area: Nomalized Area, NA: A / n∑i=1 Ai x 100 n = highest level of substitution Determine the average degree of substitution: Average Degree of Substitution = n∑i=1 (Level of Substitution for Peak x NA) / 100 Acceptance criteria: 6.2~6.9 for average degree of substitution For each of betadex sulfobutyl ether sodium peaks I–X, see limit range (% peak area) in Table 7. Table 7 | Betadex Sulfobutyl Ether Sodium Peaks I–X | Limit Range (% Peak Area) |
| I | 0-0.3 |
| II | 0-0.9 |
| III | 0.5-5.0 |
| IV | 2.0-10.0 |
| V | 10.0-20.0 |
| VI | 15.0-25.0 |
| VII | 20.0-30.0 |
| VIII | 10.0-25.0 |
| IX | 2.0-12.0 |
| X | 0-4.0 |
• PH <791>: 4.0-6.8, in a 30% (w/v) solution in carbon dioxide-free water • WATER DETERMINATION, Method I <921>: NMT 10.0% ADDITIONAL REQUIREMENTS • PACKAGING AND STORAGE: Preserve in well-closed containers, and store at room temperature. Protect from moisture. • LABELING: Label it to indicate its use in the manufacture of injectable dosage forms. • USP REFERENCE STANDARDS <11> USP Beta Cyclodextrin RS USP Betadex Sulfobutyl Ether Sodium RS USP Endotoxin RS USP Sodium Chloride RS■1S (NF30)182410-00-0 - Safety:
Betadex Sulfobutyl Ether Sodium is derived from b-cyclodextrin, which is nephrotoxic when administered parenterally. However, studies have shown that sulfobutylether bcyclodextrin is well tolerated at high doses, when administered via intravenous bolus injections, orally, and by inhalation. Up to 9 g/day may be administered by IV infusion in a licensed voriconazole formulation. The safety following high doses of sulfobutylether β-cyclodextrin intravenous administration in humans is continually being investigated. Betadex Sulfobutyl Ether Sodium has been subjected to an extensive battery of in vitro and in vivo genotoxicity and pharmacological evaluations. No genotoxic or mutagenic changes were observed with Betadex Sulfobutyl Ether Sodium administration. Betadex Sulfobutyl Ether Sodium is biocompatible and exhibits no pharmacological activity. It is rapidly eliminated unmetabolized when administered intravenously.182410-00-0 - Regulatory Status:
Betadex Sulfobutyl Ether Sodium is included in IV and IM injectable products currently approved and marketed in the USA, Europe, and Japan. It is included in the FDA Inactive Ingredients Database for IM and IV use. Its use by other routes, including SC, oral, inhalation, nasal and ophthalmic, is being evaluated in clinical studies.182410-00-0 - Preparation:
Using β-cyclodextrin and 1,4-sulfobutyrolactone as raw materials, by introducing an appropriate amount of organic solvent into the alkaline aqueous solution, the solubility of 1,4-sulfobutyrolactone is increased, and the synthesis yield of sulfobutyl ether-β-cyclodextrin is improved; the obtained product solution is subjected to ultrasonic dialysis, activated carbon decolorization, freeze drying and other operations to obtain sulfobutyl ether-β-cyclodextrin powder products.