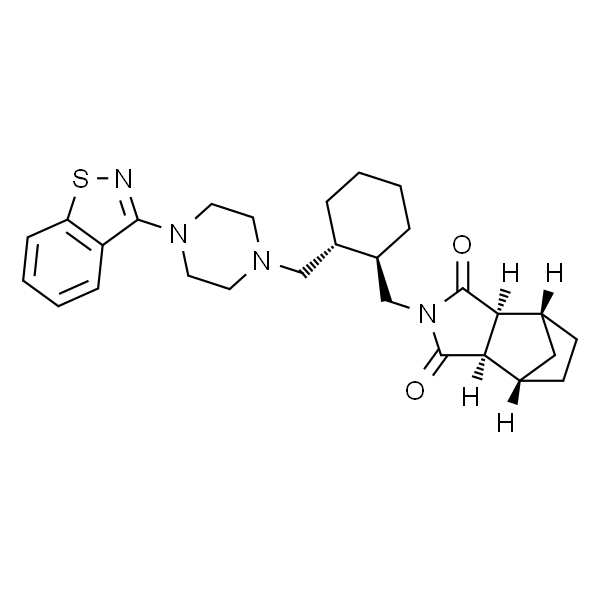
Chemical Properties:
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply Lurasidone Hydrochloride Related Intermediates: (1R,2R)-1,2-Cyclohexanedimethanol CAS 65376-05-8 (3aR,4S,7R,7aS)-rel-Hexahydro-4,7-methano-1H-isoindole-1,3(2H)-dione CAS 14805-29-9 Lurasidone Hydrochloride CAS 367514-88-3 API| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| NMR | Consistent with Structure |
| Solubility | Meets the Requirements |
| Max. Single Impurity | ≤0.20% |
| Total Impurities | ≤0.50% |
| Moisture (K.F) | ≤1.0% |
| Residue of Ignition | ≤0.20% |
| Heavy Metals | ≤20ppm |
| Purity | ≥99.0% |
| Test Standard | Enterprise Standard |
| Usage | API, Atypical Antipsychotic Drug (AAPD) |
Description:
Specifications:
Package & Storage:
| Chemical Name | Lurasidone Hydrochloride |
| Synonyms | Lurasidone HCl; Latuda |
| CAS Number | 367514-88-3 |
| CAT Number | RF-API64 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C28H36N4O2S |
| Molecular Weight | 492.684 |
| Melting Point | 198.0~205.0°C |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Lurasidone Hydrochloride (CAS 367514-88-3) with high quality, API. Lurasidone Hydrochloride (CAS 367514-88-3) is an orally administered substance that belongs to the class of atypical antipsychotic drugs (AAPD), developed by Dainippon Sumitomo Pharma. It was approved by the U.S. Food and Drug Administration (FDA) for treatment of schizophrenia on October 29, 2010 and is currently pending approval for the treatment of bipolar disorder in the United States. It received approval by the EMA in 2014 for schizophrenia. Lurasidone has also been launched in a number of countries outside the US and EU, including Canada, where it is approved for both indications. It is produced through a multi-step chemical process.