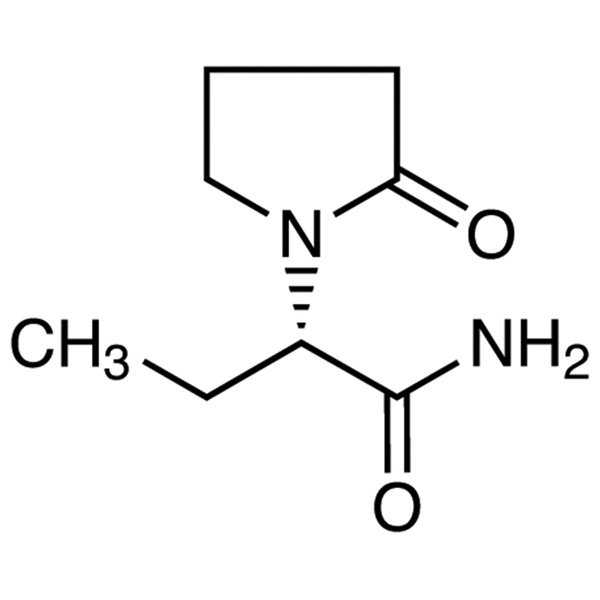
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply with High Purity and Stable Quality Chemical Name: Levetiracetam CAS: 102767-28-2 API USP/EP Standard, Commercial Production Third-generation Antiepileptic Drug| Item | Specifications |
| Appearance | White or Almost White Crystals Powder |
| Identification IR | The Spectrum abtained from sample consists with that obtained from reference substance |
| Appearance of Solution | Clear and not more intensely coloured than BY6 |
| Enantiomeric Purity Impurity D | ≤0.80% |
| Water | ≤0.50% |
| Sulfated Ash | ≤0.10% |
| Heavy Metals | ≤0.001% |
| Related Substances | |
| Impurity A | ≤0.30% |
| Any Unspecified Impurity | ≤0.05% |
| Total Unspecified Impurities | ≤0.10% |
| Total Impurities | ≤0.40% |
| Impurity F | ≤0.10% |
| Residual Solvents | |
| Benzene | ≤2ppm |
| Dichloromethane | ≤600ppm |
| Ethyl Acetate | ≤5000ppm |
| Acetone | ≤5000ppm |
| Assay | 98.0%~102.0% |
| Test Standard | USP Standard; EP Standard |
| Usage | API Third-generation Antiepileptic Drug |
Description:
Specifications:
Package & Storage:
| Chemical Name | Levetiracetam |
| Synonyms | LEV; (S)-2-(2-Oxo-1-pyrrolidinyl)butyramide; UCB-L059 |
| CAS Number | 102767-28-2 |
| CAT Number | RF-API61 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H14N2O2 |
| Molecular Weight | 170.21 |
| Melting Point | 116.0-119.0℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Levetiracetam (CAS: 102767-28-2), a derivative of pilacetam, is a novel third-generation antiepileptic drug approved by the US FDA in 1999. It was initially used for the adjunctive treatment of partial seizures in adults. In 2005, Levetiracetam was approved in oral tablets and solutions for the adjunctive treatment of partial seizures in children aged 4 years and older. It is mainly used for the additive treatment of partial seizures in adults and children over 4 years old, and can also be used only for partial seizures and systemic seizures in adults. It also has certain curative effect on myoclonic epilepsy in teenagers, refractory epilepsy, absent epilepsy in children and persistent epilepsy.