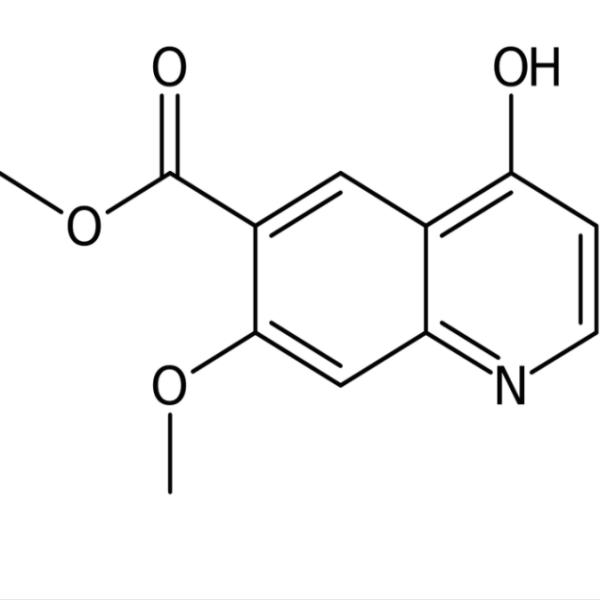
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureRuifu Chemical Supply Lenvatinib Mesylate Intermediates With High Purity Lenvatinib Mesylate CAS 857890-39-2 4-Chloro-7-Methoxyquinoline-6-Carboxamide CAS 417721-36-9 Desquinolinyl Lenvatinib; 1-(2-Chloro-4-Hydroxyphenyl)-3-Cyclopropylurea CAS 796848-79-8 Methyl 7-Methoxy-4-Oxo-1,4-Dihydroquinoline-6-Carboxylate CAS 205448-65-3 Methyl 4-Amino-2-Methoxybenzoate CAS 27492-84-8 5-(Methoxymethylene)-2,2-Dimethyl-1,3-Dioxane-4,6-Dione CAS 15568-85-1 4-Amino-3-Chlorophenol CAS 17609-80-2 4-Amino-3-Chlorophenol Hydrochloride CAS 52671-64-4 Methyl 4-Chloro-7-Methoxyquinoline-6-Carboxylate CAS 205448-66-4| Item | Specifications |
| Appearance | Off-White to Yellowish Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Loss on Drying | <1.00% |
| Residue on Ignition | <0.50% |
| Total Impurities | <2.00% |
| H-NMR | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Description:
Specifications:
Package & Storage:
| Chemical Name | Methyl 7-Methoxy-4-Oxo-1,4-Dihydroquinoline-6-Carboxylate |
| Synonyms | 1,4-Dihydro-7-Methoxy-4-Oxo-6-Quinolinecarboxylic Acid Methyl Ester; 7-Methoxy-4-Oxo-1,4-Dihydro-Quinoline-6-Carboxylic Acid Methyl Ester; Lenvatinib Intermediate 3 |
| CAS Number | 205448-65-3 |
| CAT Number | RF-PI1973 |
| Stock Status | In Stock, Production Capacity 50 MT/Year |
| Molecular Formula | C12H11NO4 |
| Molecular Weight | 233.22 |
| Boiling Point | 421.0±45.0℃ |
| Density | 1.267±0.060 g/cm3 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Methyl 7-Methoxy-4-Oxo-1,4-Dihydroquinoline-6-Carboxylate (CAS: 205448-65-3) is an intermediate of Lenvatinib Mesylate (CAS: 857890-39-2). Lenvatinib, sold under the brand name Lenvima among others, is an anti-cancer medication for the treatment of certain kinds of thyroid cancer and for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases. Lenvatinib is approved (since 2015) for the treatment of differentiated thyroid cancer that is either locally recurrent or metastatic, progressive, and did not respond to treatment with radioactive iodine (radioiodine). In May 2016, the U.S. Food and Drug Administration (FDA) approved it (in combination with everolimus) for the treatment of advanced renal cell carcinoma following one prior anti-angiogenic therapy. The drug is also approved in the US and in the European Union for hepatocellular carcinoma that cannot be removed surgically in patients who have not received cancer therapy by mouth or injection.