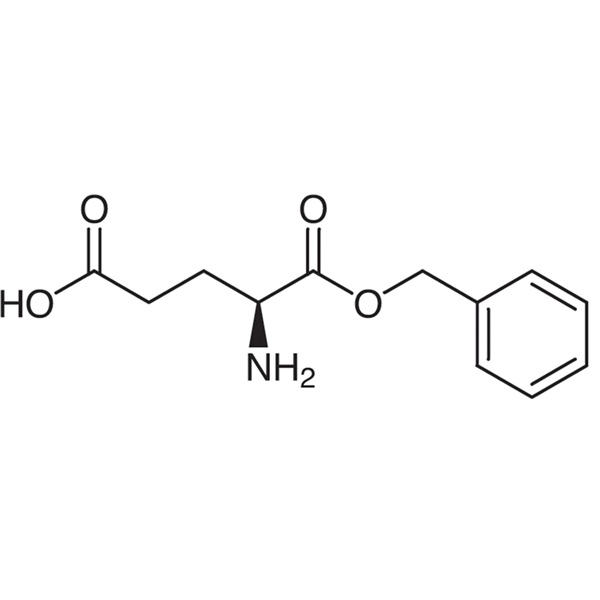
Chemical Properties:
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light and moisture.Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of L-Glutamic Acid α-Benzyl Ester (H-Glu-OBzl) (CAS: 13030-09-6) with high quality. Ruifu Chemical supplys a series of amino acids and derivatives. We can provide worldwide delivery, small and bulk quantities available. If you need L-Glutamic Acid α-Benzyl Ester, Please contact: alvin@ruifuchem.comDescription:
Package & Storage:
| Chemical Name | L-Glutamic Acid α-Benzyl Ester |
| Synonyms | H-Glu-OBzl; H-L-Glu-OBzl; L-Glutamic Acid 1-Benzyl Ester; 1-Benzyl L-Glutamate; L-Glutamic Acid alpha-Benzyl Ester; (S)-4-Amino-5-(Benzyloxy)-5-Oxopentanoic Acid |
| Stock Status | In Stock |
| CAS Number | 13030-09-6 |
| Molecular Formula | C12H15NO4 |
| Molecular Weight | 237.26 |
| Melting Point | 140.0~150.0℃ |
| Density | 1.245±0.06 g/cm3 |
| Sensitive | Air Sensitive, Moisture Sensitive, Heat Sensitive |
| Storage Temp. | Sealed in Dry, Store at Room Temperature |
| COA & MSDS | Available |
| Classification | Amino Acids & Derivatives |
| Brand | Ruifu Chemical |
Advantages:
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com 15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals. Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc. Advantages? Superior quality, affordable price, professional services and technical support, fast delivery. Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc. Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers. Factory Audit? Factory audit welcome. Please make an appointment in advance. MOQ? No MOQ. Small order is acceptable. Delivery Time? If within stock, three days delivery guaranteed. Transportation? By Express (FedEx, DHL), by Air, by Sea. Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided. Custom Synthesis? Can provide custom synthesis services to best fit your research needs. Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.FAQ:
Stable Supply: Maintain reasonable stock
Professional Service: One stop purchasing service
Technical Support: Technology solution available
Custom Synthesis Service: Ranged from grams to kilos
Fast Delivery: If within stock, three days delivery guaranteed
High Quality: Established a complete quality assurance system
Sufficient Capacity: Sufficient facilities and technicians
OEM Package: Custom package and label available
Specifications:
| Items | Inspection Standards | Results |
| Appearance | White or Off-White Crystalline Powder | Conforms |
| Loss on Drying | <0.50% | 0.24% |
| Melting Point | 140.0~150.0℃ | 146.0~150.0℃ |
| Assay / Analysis Method | >98.5% (HPLC) | 98.84% |
| Specific Rotation [α]20/D | +10.0 to +12.0° (C=1, 1mol/L HCl) | Conforms |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Proton NMR Spectrum | Conforms to Structure | Conforms |
| Conclusion | This Product by Inspection Accords with the Enterprise Standard | |
| Main Uses | Amino Acids and Derivatives; Pharmaceutical Intermediates | |
Application:
L-Glutamic Acid α-Benzyl Ester (H-Glu-OBzl) (CAS: 13030-09-6), Amino Acids and Derivatives; Pharmaceutical Intermediates.Process of Production:
1. L-Glutamic Acid, Sodium Hydroxide, Water, Acetone, Boc-Anhydride 2. Boc-L-Glu-OH reacted with Benzyl Bromide and Triethylamine to obtain Boc-Glu-OBzl 3. HCL/EA Take off Boc-, Adjust the PH, Obtain H-Glu-OBzl 4. Solvents Used: DMF, Ethyl Acetate, EthanolStructures of Impurities:
Package & Storage:
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light and moisture.Method of Analysis:
1. Purity (HPLC) 1.1 Instruments High performance liquid chromatography, UV3000 detector. Electronic analytical balance 1.2 Reagents Acetonitrile (chromatographic grade), TFA (chromatographic grade) 1.3 Chromatographic conditions 1.3.1 Column: C18 column, 5 μl * 250 * 4.6 mm 1.3.2 Detection wavelength: 220nm Flow rate: 1.0 mL/min Injection volume:10 μL(for reference) Diluent: acetonitrile Run time: 20 minutes 1.4 Mobile phase preparation Mobile phase A (0.1% Trifluoroacetic acid Water): accurately absorb 1.0 ml TFA diluted to 1000 ml with water, mix and degas. Mobile phase B (0.1% Trifluoroacetic acid Acetonitrile): accurately absorb 1.0 ml TFA, diluted to 1000 ml with acetonitrile, mix and degas. 1.5 Gradient Program Time (min) A% B% 0:00 90 10 10.00 10 90 15.00 10 90 15.01 90 10 20.00 90 10 1.6 Preparation of sample solution Weigh 0.1 g sample and dissolve it with acetonitrile and dilute it to 100ml. Shake well for future use, or the same concentration. Prepare two samples in parallel. 1.7 Sample Determination Analyze the sample according to the following sampling procedure: 1) More than 1 injection of blank solution 2) 1 injection of sample solution 1# 3) 1 injection of sample solution 2# 1.8.0 Calculations 1.8.1 The peak area normalization method was used to calculate the purity of HPLC. 1.8.2 The relative mean deviation of the purity of two needles shall not be greater than 1% 1.8.3 If the results of both injections meet the acceptance criteria, take the average purity as the final result.