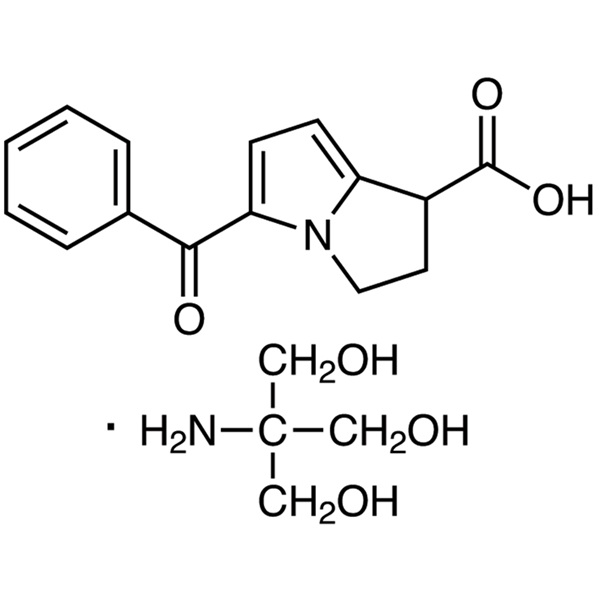
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in a tightly closed container. Store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery. Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Ketorolac Tromethamine Salt (CAS: 74103-07-4) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| pH | 5.7~6.7 |
| Melting Point | 160.0~161.0℃ |
| Purity / Analysis Method | >99.0% (HPLC) |
| Assay / Analysis Method | 98.5~101.5% (Calculated on the Dried Basis) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.10% |
| Heavy Metals (Pb) | ≤20ppm |
| Related Substances | |
| Impurity RRT0.54 | <0.50% |
| Impurity RRT0.66 | <0.50% |
| Ketorolac 1-Keto Analog | <0.10% |
| Ketorolac 1-Hydroxy Analog | <0.10% |
| Any Other Single Impurity | <0.20% |
| Total Impurities | <1.00% |
| Residual Solvents | |
| Dichloroethane | <50ppm |
| Anhydrous Ethanol | <5000ppm |
| Microorganism Limits | |
| Bacteria Amount | <1000cfu/g |
| Mould and Yeast Amount | <100cfu/g |
| Escherichia. Coli | Absent |
| Bacterial Endotoxin | <5EU/mg |
| Infrared Spectrum | Conforms to Structure |
| Solubility in H2O | Colorless to Faint Yellow Clear (15 mg/ml) Pass |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Ketorolac Tromethamine |
| Synonyms | Ketorolac Tris Salt; Ketorolac (Tromethamine Salt); rac Ketorolac Tromethamine Salt; Toradol; (±)-5-Benzoyl-2,3-Dihydro-1H-Pyrrolizine-1-Carboxylic Acid Tris Salt; (±)-Form Tromethamine Salt |
| CAS Number | 74103-07-4 |
| Stock Status | In Stock, Commercially Manufactured |
| Molecular Formula | C15H13NO3·C4H11NO3 |
| Molecular Weight | 376.41 |
| Melting Point | 160.0~161.0℃ |
| Boiling Point | 493.2℃ at 760 mmHg |
| Sensitive | Hygroscopic. Light Sensitive |
| λmax | 322nm(MeOH)(lit.) |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
Advantages:
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com 15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals. Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc. Advantages? Superior quality, affordable price, professional services and technical support, fast delivery. Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc. Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers. Factory Audit? Factory audit welcome. Please make an appointment in advance. MOQ? No MOQ. Small order is acceptable. Delivery Time? If within stock, three days delivery guaranteed. Transportation? By Express (FedEx, DHL), by Air, by Sea. Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided. Custom Synthesis? Can provide custom synthesis services to best fit your research needs. Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.FAQ:
Stable Supply: Maintain reasonable stock
Professional Service: One stop purchasing service
Technical Support: Technology solution available
Custom Synthesis Service: Ranged from grams to kilos
Fast Delivery: If within stock, three days delivery guaranteed
High Quality: Established a complete quality assurance system
Application:
Ketorolac Tromethamine Salt (CAS: 74103-07-4) is a nonsteroidal antiinflammatory agent that exhibits analgesic and antipyretic activity. It is a non-selective COX inhibitor with IC50s of 20 nM for both COX-1 and COX-2. Ketorolac Tromethamine Salt is effective in the management of moderate to severe postoperative pain. It is, however, the first of this type of agent to be administered parenterally as an analgesic and is specifically indicated for intramuscular injection. Ketorolac represents a useful alternative to the narcotic analgesics due to its lack of abuse potential. It is used primarily for its analgesic effects for short-term treatment of mild to moderate pain in dogs and rodents. The duration of analgesic effect in dogs is about 8-12 hours, but because of the availability of approved, safer NSAIDs for dogs, its use is questionable. An organoammonium salt resulting from the mixture of equimolar amounts of ketorolac and tromethamine (tris). It has potent non-sedating analgesic and moderate anti-inflammatory effects. It is used in the short-term management of post-operative pain, and in eye drops to relieve the ocular itching associated with seasonal allergic conjunctivitis.Sufficient Capacity: Sufficient facilities and technicians
OEM Package: Custom package and label available
74103-07-4 - Risk and Safety:
Hazard Symbols T - Toxic Risk Codes R25 - Toxic if swallowed R36/37/38 - Irritating to eyes, respiratory system and skin. R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed. Safety Description S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. UN IDs UN 2811 6.1/PG 3 WGK Germany 3 RTECS UY7759900 HS Code 2933995500 Hazard Class 6.1(a) Packing Group II74103-07-4 - USP 35 Analysis Method:
Ketorolac Tromethamine contains not less than 98.5 percent and not more than 101.5 percent of C15H13NO3·C4H11NO3, calculated on the dried basis. Packaging and storage-Preserve in tight, light-resistant containers. Store at 25°, excursions permitted between 15° and 30° USP Reference standards <11>- USP Ketorolac Tromethamine RS Identification- A: Infrared Absorption <197K>. B: Ultraviolet Absorption <197U>- Solution: 10 µg per mL Medium: methanol. C: Tromethamine test-Prepare a Standard solution of USP Ketorolac Tromethamine RS in a mixture of dichloromethane and methanol (2:1) containing 5 mg per mL. Similarly prepare a test solution of Ketorolac Tromethamine containing 5 mg per mL. Apply 40-µL volumes of the Standard solution and the test solution to a thin-layer chromatographic plate (see Chromatography <621>) coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a chromatographic chamber previously equilibrated with a mixture of dichloromethane, acetone, and glacial acetic acid (95:5:2). Seal the chamber, and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, and allow the solvent to evaporate. Spray the plate with a freshly prepared alcoholic solution containing 30 mg of ninhydrin per mL, and heat the plate at about 150° for 2 to 5 minutes. Yellow spots with pink to purple borders develop on the plate in the areas where the Standard solution and the test solution were applied. pH <791>: between 5.7 and 6.7, in a solution (1 in 100). Loss on drying <731>-Dry it in vacuum at 60° for 3 hours: it loses not more than 0.5% of its weight. Residue on ignition <281>: not more than 0.1%. Heavy metals, Method II <231>: 0.002%. Chromatographic purity- Mobile phase, Solvent mixture, Standard preparation, Resolution solution, and Chromatographic system-Proceed as directed in the Assay. Test solution-Use the Assay preparation. Procedure-Chromatograph the Test solution as directed for Procedure in the Assay, allowing the chromatography to extend to three times the retention time of ketorolac. Measure the responses of all the peaks. Calculate the percentage of each individual impurity in the portion of Ketorolac Tromethamine taken by the formula: 100rfi (ri / rs) in which rfi is the response factor of each individual impurity peak relative to that of ketorolac; ri is the peak response for each impurity; and rs is the sum of all the peak responses of the impurity peaks and the major ketorolac peak. The rfi values are 0.52 for the ketorolac 1-keto analog, 0.67 for the ketorolac 1-hydroxy analog, 2.2 for the impurity peak having a retention time of 0.54 relative to that of ketorolac, and 0.91 for the impurity peak at a relative retention time of 0.66. Not more than 0.1% of the ketorolac 1-keto analog or of the ketorolac 1-hydroxy analog is found; not more than 0.5% of any other impurity is found; and the sum of all impurities is not more than 1.0%. Assay- Mobile phase-Dissolve 5.75 g of monobasic ammonium phosphate in 1000 mL of water, and adjust with phosphoric acid to a pH of 3.0. Prepare a filtered and degassed mixture of this buffer solution and tetrahydrofuran (70:30). Make adjustments if necessary (see System Suitability under Chromatography <621>) to achieve a retention time for ketorolac of about 8 to 12 minutes. Solvent mixture-Prepare a mixture of water and tetrahydrofuran (70:30). Standard preparation-Quantitatively dissolve an accurately weighed quantity of USP Ketorolac Tromethamine RS in Solvent mixture to obtain a solution having a known concentration of about 0.4 mg per mL. [NOTE-Protect this solution from light.] Assay preparation-Transfer about 20 mg of Ketorolac Tromethamine, accurately weighed, to a 50-mL volumetric flask, dilute with Solvent mixture to volume, and mix. [NOTE-Protect this solution from light.] Resolution solution-In a 250-mL separator mix 100 mL of water, 100 mL of dichloromethane, 30 mg of USP Ketorolac Tromethamine RS, and 1 mL of 1 N hydrochloric acid. Insert the stopper, shake, and allow the layers to separate. Transfer the lower dichloromethane layer to a stoppered borosilicate glass flask, and discard the upper layer. Expose the dichloromethane solution to direct sunlight for 10 to 15 minutes. Transfer 1.0 mL of the solution to a vial, evaporate in a current of air or in a stream of nitrogen to dryness, add 1.0 mL of Solvent mixture, and swirl to dissolve. [NOTE-This solution may be stored under refrigeration and used as long as the chromatogram obtained as directed for Procedure is suitable for identifying the peaks due to the ketorolac 1-keto analog and ketorolac 1-hydroxy analog, and for the measurement of the resolution between the ketorolac 1-keto analog and ketorolac.] Chromatographic system (see Chromatography <621>)—The liquid chromatograph is equipped with a 313-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7 and is maintained at a constant temperature of about 40°. The flow rate is about 1.5 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.63 for the ketoroac 1-hydroxy analog, 0.89 for the ketorolac 1-keto analog, and 1.0 for ketorolac; and the resolution, R, between the ketorolac 1-keto analog and ketorolac is not less than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency is not less than 5500 theoretical plates; and the relative standard deviation for replicate injections is not more than 1.5%. Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C15H13NO3·C4H11NO3 in the portion of Ketorolac Tromethamine taken by the formula: 50C(rU / rS) in which C is the concentration, in mg per mL, of USP Ketorolac Tromethamine RS in the Standard preparation; and rU and rS are the ketorolac peak responses obtained from the Assay preparation and the Standard preparation, respectively.