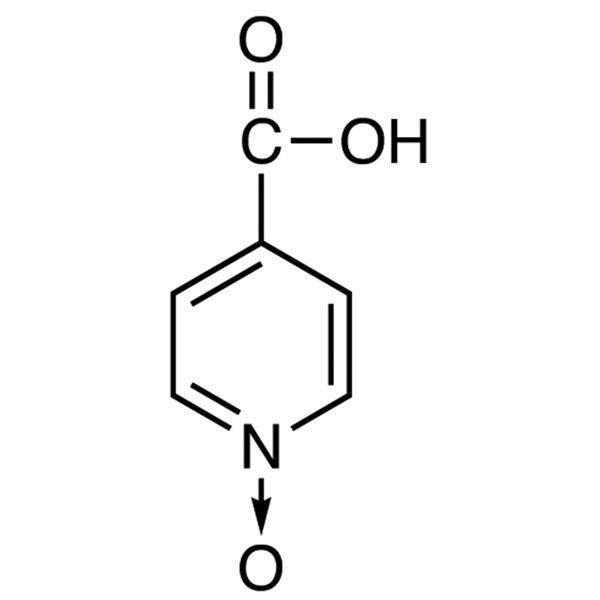
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Isonicotinic Acid N-Oxide (CAS: 13602-12-5) with high quality, commercial production. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | White to Off-White Powder or Crystals |
| Purity / Analysis Method | >98.0% (HPLC) |
| Melting Point | 265.0~271.0℃ |
| Loss on Drying | <0.50% |
| Heavy Metals | <20ppm |
| Total Impurities | <2.00% |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Isonicotinic Acid N-Oxide |
| Synonyms | Isonicotinic Acid 1-Oxide; Pyridine-4-Carboxylic Acid N-Oxide; Pyridine-4-Carboxylic Acid 1-Oxide; 4-Carboxypyridine 1-Oxide |
| CAS Number | 13602-12-5 |
| CAT Number | RF-PI1862 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H5NO3 |
| Molecular Weight | 139.11 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Isonicotinic Acid N-Oxide (CAS: 13602-12-5) interacts with 3d metal(II) perchlorates in ethanol-triethyl orthoformate leading to partial substitution of perchlorate with isonicotinate-N-oxide anionic groups. It reacts with AgClO4 or AgBF4 to yield distinct crystalline products. It forms polymeric lanthanide(III) complexes. Isonicotinic acid N-oxide was used in the synthesis of three-dimensional coordination polymers based on inorganic lanthanide(II) sulfate skeletons. Isonicotinic acid N-oxide interacts with 3d metal(II) perchlorates in ethanol-triethyl orthoformate leading to partial substitution of perchlorate with isonicotinate-N-oxide anionic groups. It reacts with AgClO4 or AgBF4 to yield distinct crystalline products. It forms polymeric lanthanide(III) complexes.