Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureManufacturer Supply, High Purity, Commercial Production| Item | Specifications |
| Characteristic | White to Off-White Crystalline Powder, Slightly soluble in alcohol and in methylene chloride, practically insoluble in water |
| Identification Infrared Absorption | The IR spectrum is in accordance with the spectrum obtained with Irbesartan RS |
| Identification 2 | The retention time of the major peak in the chromatogram of the assay corresponds to Irbesartan RS |
| Purity / Analysis Method | >99.5% (HPLC) |
| Water | <0.50% |
| Heavy Metals | <0.002% |
| Residue on Ignition | <0.10% |
| Related Substances | (By HPLC) |
| USP Impurity A | <0.20% |
| Any Unidentified Impurity | <0.10% |
| Total Impurities | <0.50% |
| Organic VolatileImpurities | Meets the requirements of USP |
| Residual Azide | <10ppm |
| Residual Solvents | (By GC) |
| Ethanol | <5000ppm |
| Toluene | <890ppm |
| Dichloromethane | <3000ppm |
| N,N-Dimethylformamide | <880ppm |
| T-Butyl Methyl Ether | <5000ppm |
| Assay | 98.0~102.0% (calculated on anhydrous basis) |
| Test Standard | Enterprise Standard; USP Standard |
| Usage | API; Antihypertensive; For the treatment of hypertension |
Description:
Specifications:
Package & Storage:
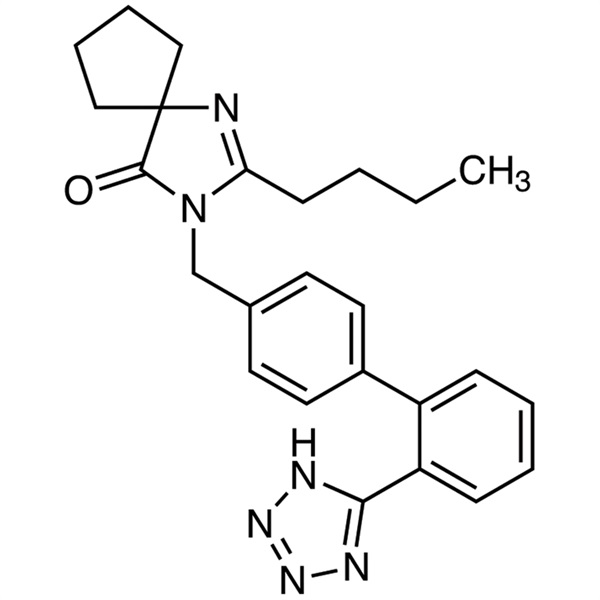
| Chemical Name | Irbesartan |
| Synonyms | BMS-186295; SR-47436; Aprovel; Avapro; 2-Butyl-3-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one; 2-Butyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one |
| CAS Number | 138402-11-6 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C25H28N6O |
| Molecular Weight | 428.54 |
| Density | 1.30±0.10 g/cm3 |
| Solubility in Water | Insoluble in Water |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Irbesartan (CAS: C) is an angiotensin II receptor antagonist used mainly for the treatment of hypertension. Irbesartan was developed by Sanofi Research (now part of Sanofi-Aventis). It is jointly marketed by Sanofi-Aventis and Bristol-Myers Squibb under the trade names Aprovel, Karvea, and Avapro. Irbesartan is used to treat high blood pressure. Avapro was launched in Germany, the UK and the US for hypertension. As with all angiotensin II receptor antagonists, irbesartan is indicated for the treatment of hypertension. Irbesartan may also delay progression of diabetic nephropathy and is also indicated for the reduction of renal disease progression in patients with type 2 diabetes, hypertension and microalbuminuria (>30 mg/24 hours) or proteinuria. Irbesartan can also reduce electrical remodeling of the myocardium, thereby reduce the mortality rate of patients with hypertension, it is the most effective drug for treatment of hypertension and cardiovascular disease.