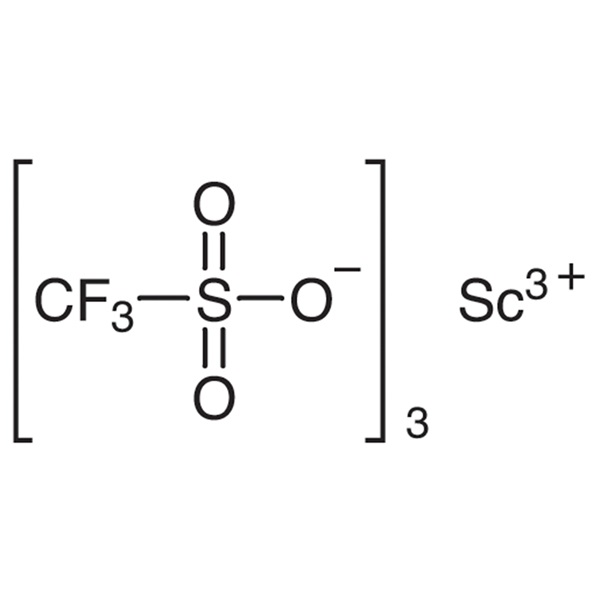
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Scandium(III) Trifluoromethanesulfonate (CAS: 144026-79-9) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | White Powder |
| Purity / Analysis Method | >98.0% (Chelometric Titration) |
| Scandium | >9.0% (Complexometric EDTA) |
| Infrared Spectrum | Conforms to Structure |
| X-Ray Diffraction | Conforms to Structure |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Scandium(III) Trifluoromethanesulfonate |
| Synonyms | Scandium(III) Triflate; Scandium Trifluoromethanesulfonate; Trifluoromethanesulfonic Acid Scandium(III) Salt; Sc(OTf)3 ; Sc(SO3CF3)3 |
| CAS Number | 144026-79-9 |
| CAT Number | RF-PI2108 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | Sc(SO3CF3)3 |
| Molecular Weight | 492.16 |
| Sensitivity | Hygroscopic |
| Melting Point | >300℃ |
| Solubility | Soluble in Water, Alcohol and Acetonitrile |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Scandium(III) Trifluoromethanesulfonate, also known as Scandium(III) Triflate, (CAS: 144026-79-9) is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) Trifluoromethanesulfonate is widely used as a catalyst in hydrothiolation, selective two-electron reduction of oxygen by ferrocene derivatives and vinylogous Fridel-crafts alkylation of indoles and pyrrole in water. It is involved in the Mukaiyama aldol addition and stereochemically catalyzes the radical polymerization of acrylates. It acts as a Lewis acid catalyst and used in the synthesis of bullvalone via a stabilized sulfur ylide. Reactions: Water tolerant Lewis acid. Commonly used in a range of Lewis acid catalyzed reactions. Efficient metal source for Lewis acid catalyzed asymmetric reactions. Catalyzes Friedel-Crafts alkylation, acylation and related reactions. Catalyzes various domino- and multi-component processes. Catalyzes electrophilic additions of alpha-diazoesters with ketones. Catalyzes carbon insertion reactions.