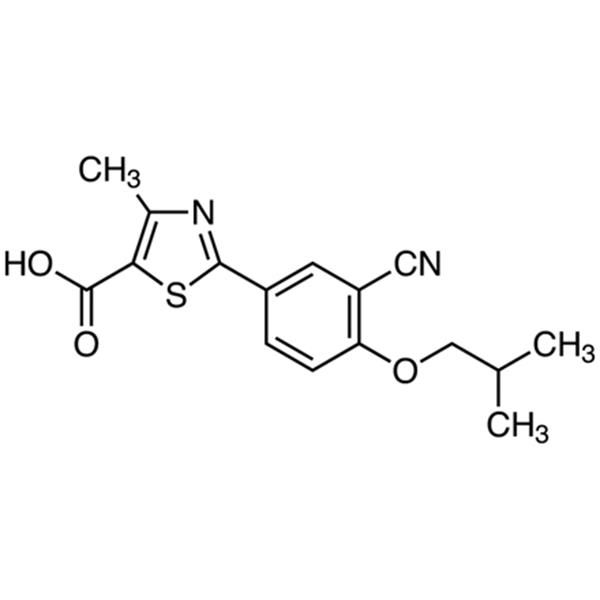
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureRuifu Chemical Supply Febuxostat Related Intermediates: Febuxostat CAS 144060-53-7 Ethyl 2-Chloroacetoacetate CAS 609-15-4 4-Hydroxythiobenzamide CAS 25984-63-8 Ethyl 2-(3-Cyano-4-Hydroxyphenyl)-4-Methyl-1,3-Thiazole-5-Carboxylate CAS 161798-02-3 Ethyl 2-(3-Cyano-4-Isobutoxyphenyl)-4-Methyl-5-Thiazolecarboxylate CAS 160844-75-7 Ethyl 2-(3-Formyl-4-Hydroxyphenyl)-4-Methylthiazole-5-Carboxylate CAS 161798-01-2 Ethyl 2-(3-Formyl-4-Isobutoxyphenyl)-4-Methylthiazole-5-Carboxylate CAS 161798-03-4 Ethyl 2-(4-Hydroxyphenyl)-4-Methylthiazole-5-Carboxylate CAS 161797-99-5| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Identification | HPLC/IR |
| Purity / Analysis Method | >99.0% (HPLC on dried basis) |
| Loss on Drying | <1.00% (Dry it at 105℃ in an electric air blowing dryer till a constant weight) |
| Residues on Ignition | <0.10% |
| Related Substances | |
| Maximum Single Impurity | <0.30% |
| Total Impurities | <1.00% |
| Chlorides | <0.04% |
| Heavy Metals | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | API |
Description:
Specifications:
Package & Storage:
| Chemical Name | Febuxostat |
| Synonyms | 2-(3-Cyano-4-Isobutoxyphenyl)-4-Methylthiazole-5-Carboxylic Acid |
| CAS Number | 144060-53-7 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C16H16N2O3S |
| Molecular Weight | 316.38 |
| Melting Point | 207.0 to 211.0℃ |
| Density | 1.31±0.1 g/cm3 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Febuxostat (CAS: 144060-53-7), sold under the brand names Uloric and Adenuric among others, is a medication used long-term to treat gout due to high uric acid levels. It is generally recommended only for people who cannot take allopurinol. When initially started, medications such as NSAIDs are often recommended to prevent gout flares. It is taken by mouth. Febuxostat was approved for medical use in the European Union in 2008 and in the United States in 2009. Febuxostat is used to treat chronic gout and hyperuricemia. Febuxostat is typically recommended only for people who cannot tolerate allopurinol.