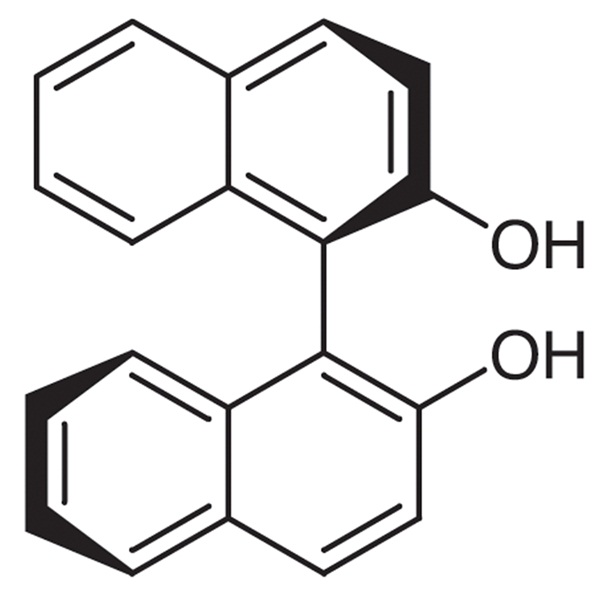
Chemical Properties:
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply with High Purity and Stable Quality 1,1'-Bi-2-naphthol CAS 602-09-5 (R)-(+)-1,1'-Bi-2-naphthol; (R)-(+)-BINOL; CAS 18531-94-7 (S)-(-)-1,1'-Bi-2-naphthol; (S)-(-)-BINOL; CAS 18531-99-2 (R)-(-)-1,1'-Binaphthyl-2,2'-diyl Hydrogen Phosphate; (R)-(-)-BNP Acid; CAS 39648-67-4 (S)-(+)-1,1'-Binaphthyl-2,2'-diyl Hydrogen Phosphate; (S)-(+)-BNP Acid; CAS 35193-64-7 Chiral Compounds, High Quality, Commercial Production| Item | Specifications |
| Appearance | White Crystalline Powder |
| Loss on Drying | ≤0.50% |
| Melting Point | 208.0~210.0℃ |
| Specific Rotation [a]22/D | +34.0° ~ +37.0° (C=1 in THF) |
| Chiral Assay | e.e ≥99.5% (HPLC) Chiral Column |
| Chemical Assay | ≥99.5% (HPLC) |
| Test Standard | Enterprise Standard |
| Usage | Chiral Compounds; Pharmaceutical Intermediates |
Description:
Specifications:
Package & Storage:
| Chemical Name | (R)-(+)-1,1'-Bi-2-naphthol |
| Synonyms | (R)-(+)-BINOL |
| CAS Number | 18531-94-7 |
| CAT Number | RF-CC216 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C20H14O2 |
| Molecular Weight | 286.32 |
| Density | 1.301 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (R)-(+)-1,1'-Bi-2-naphthol (CAS: 18531-94-7) with high quality, widely used in organic synthesis, synthesis of pharmaceutical intermediates and fine chemical synthesis. Shanghai Ruifu Chemical Co., Ltd. plays an important role in the chiral chemistry, the company is committed to the production of chiral compounds. Our products are widely praised by customers. (R)-(+)-1,1'-Bi-2-naphthol (CAS: 18531-94-7) is obtained through resolution of racemic BINOL, which is usually made from 2-naphthol catalyzed by iron(III) chloride through radical reaction. (R)-(+)-1,1'-Bi-2-naphthol has been widely used for various applications: 1) chiral inducing agents for catalytic, asymmetric reactions such as the Diels-Alder reaction,ene reaction,or as Lewisacids; 2) enantioselective reduction of ketones; 3) synthesis of chiral macrocyclesand otherinteresting compounds. A chiral binapthol imminium salt precursor used in the catalytic asymmetric oxidation of sulfides to sulfoxides. Chiral lanthanide triflates formed from binaphthol serve as catalysts for asymmetric Diels-Alder reactions. Derivatives of binaphthol have recently been used in asymmetric Claisen rearrangements and asymmetric epoxidations. The lithium aluminum hydride derivative of these diols (BINAP-H) has been used extensively for the reduction of ketones. It is used in biosynthetic preparation for enantioselective oxidation of naphthols to binaphthyldiols with horseradish peroxidase catalyst.