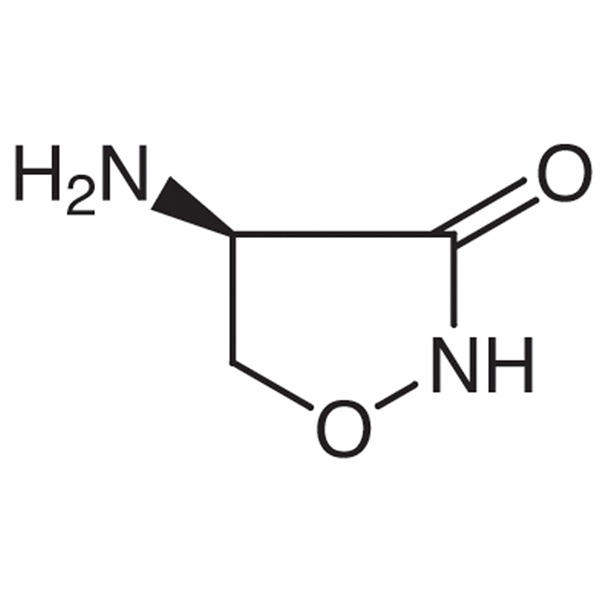
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture. Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery. Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of D-(+)-Cycloserine (CAS: 68-41-7) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase D-(+)-Cycloserine, Please contact: alvin@ruifuchem.comDescription:
Package/Storage/Shipping:
| Chemical Name | D-(+)-Cycloserine |
| Synonyms | D-Cycloserine; (+)-Cycloserine; (R)-(+)-Cycloserine; (R)-(+)-4-Amino-3-Isoxazolidinone; Orientomycin; Oxamycin; α-Cycloserine |
| Stock Status | In Stock |
| CAS Number | 68-41-7 |
| Molecular Formula | C3H6N2O2 |
| Molecular Weight | 102.09 g/mol |
| Melting Point | 137℃ |
| Sensitive | Air Sensitive, Heat Sensitive |
| Solubility | Soluble in Water. Slightly Soluble in Methanol and Propylene Glycol. Insoluble in Chloroform and Ether. |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
Advantages:
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com 15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals. Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc. Advantages? Superior quality, affordable price, professional services and technical support, fast delivery. Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc. Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers. Factory Audit? Factory audit welcome. Please make an appointment in advance. MOQ? No MOQ. Small order is acceptable. Delivery Time? If within stock, three days delivery guaranteed. Transportation? By Express (FedEx, DHL), by Air, by Sea. Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided. Custom Synthesis? Can provide custom synthesis services to best fit your research needs. Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.FAQ:
Stable Supply: Maintain reasonable stock
Professional Service: One stop purchasing service
Technical Support: Technology solution available
Custom Synthesis Service: Ranged from grams to kilos
Fast Delivery: If within stock, three days delivery guaranteed
High Quality: Established a complete quality assurance system
Sufficient Capacity: Sufficient facilities and technicians
OEM Package: Custom package and label available
Specifications:
| Items | Inspection Standards | Results |
| Appearance | White or Pale Yellowish Crystalline Powder | Complies |
| Specific Rotation [α]20/D | +108.0° to +114.0° (C=5, 2N NaOH) | +111.9° |
| Identification | A Blue Color Gradually Developed | Blue Color |
| Condensation Products | ≤0.80% (at 286nm) | 0.08% |
| Loss on Drying | <1.00% | 0.38% |
| Residue on Ignition | <0.50% | 0.10% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Volatile Impurity | ||
| - Methanol | ≤500ppm | <500ppm |
| - Acetone | ≤500ppm | <500ppm |
| Assay (Antibiotics-Microbial Assays) | ≥900μg/mg | 938μg/mg |
| pH | 5.5 to 6.5 | 5.98 |
| FTIR | Conforms | Conforms |
| IR Spectrum | Conforms | Conforms |
| NMR Spectrum | Conforms | Conforms |
| Conclusion | This product by inspection accords with the standard USP-35 | |
68-41-7 - Risk and Safety:
Application:
Hazard Symbols Xn - Harmful Risk Codes R5 - Heating may cause an explosion R20 - Harmful by inhalation Safety Description S38 - In case of insufficient ventilation, wear suitable respiratory equipment. S36/37 - Wear suitable protective clothing and gloves. S24/25 - Avoid contact with skin and eyes. WGK Germany 2 RTECS NY2975000 FLUKA BRAND F CODES 10-23 HS Code 2941909099D-(+)-Cycloserine (CAS: 68-41-7) has strong hygroscopic nature, it is soluble in water, slightly soluble in lower alcohols, acetone and dioxane, and hardly soluble in chloroform and petroleum ether. It is relatively stable in alkaline solution and decomposes rapidly in acidic or neutral solutions. As a broad-spectrum antibiotic, cycloserine is inhibitive against most Gram-positive and Gram-negative bacteria, rickettsia and some protozoa, with the exception of Mycobacterium tuberculosis., It is also effective on some of the Mycobacterium tuberculosis strains with tolerance to streptomycin, vinactane para-aminosalicylic acid, isoniazid and pyrazinamide. Cycloserine slightly synergizes with isoniazid in the inhibition of Mycobacterium tuberculosis H37RV, but it neither synergizes nor antagonizes against streptomycin. The product is a bacteriostatic agent, and thus won’t exert bactericidal effect even when increasing the dose or prolonging the action time with bacteria. The Mechanism of D-cycloserine’s antibacterial action is to inhibit the biosynthesis of peptidoglycan of the cell wall. As it is a structural analog of D-alanine, D-cycloserine can competitively inhibit the activities of alanine racemase and D-alanyl-D-alanine synthetase, which are two important enzymes in peptidoglycan synthesis. D-cycloserine shows weak inhibitive activity against Mycobacterium tuberculosis which is only 1/10 to 1/20 that of streptomycin. The advantage of the product is that it is effective on drug-resistant Mycobacterium tuberculosis strains and less likely to induce drug resistance. The product can be used with other anti-tuberculosis drugs in the treatment of tuberculosis caused by drug-resistant Mycobacterium tuberculosis. Cycloserine is a second-line anti-tuberculosis drug. It can inhibit the growth of Mycobacterium tuberculosis, but the effect is relatively weaker than that of the first-line drugs. Its efficacy in tuberculosis treatment is relatively low. Use the drug alone may produce drug resistance, but the resistance occurs slowly compared with that of other anti-tuberculosis drugs. No cross-resistance has been found between cycloserine and other anti-tuberculosis drugs. The mechanism of its antibacterial action is to inhibit the synthesis of peptidoglycan of bacterial cell wall, causing defective in cell wall architecture. The main structural component of the bacterial cell wall is peptidoglycan, which is composed of N-acetylglucosamine (GNAc) and N-acetylmuramic acid (MNAc). N-acetylmuramic acid is linked with pentapeptide and connects N-acetylglucosamine in a reduplicated and alternative manner. The formation of cytoplasmic peptidoglycan precursor may be hampered by cycloserine, as the latter can hinder the racemase and the synthetase of D-Alanine, and thus blocks the formation of N-acetylmuramic acid.USP-35 Test Method:
Cycloserine [68-41-7]. Cycloserine has a potency of not less than 900µg of C3H6N2O2 per mg. Packaging and storage-Preserve in tight containers. USP Reference standards <11>- USP Cycloserine RS Identification-Dissolve about 1 mg in 10 mL of 0.1 N sodium hydroxide. To 1 mL of the resulting solution add 3 mL of 1 N acetic acid and 1 mL of a mixture, prepared 1 hour before use, of equal parts of sodium nitroprusside solution (1 in 25) and 4 N sodium hydroxide: a blue color gradually develops. Condensation products-Its absorptivity (see Spectrophotometry and Light-Scattering <851>) at 285 nm, determined in a 0.1 N sodium hydroxide solution containing 0.40 mg per mL is not more than 0.80. Specific rotation <781S>: between 108° and 114°. Test solution: 50 mg per mL, in 2 N sodium hydroxide. Crystallinity <695>: meets the requirements pH <791>: between 5.5 and 6.5, in a solution (1 in 10). Loss on drying <731>-Dry about 100 mg in a capillar y-stoppered bottle in vacuum at 60℃ for 3 hours: it loses not more than 1.0% of its weight. Residue on ignition <281>: not more than 0.5%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid. Assay- pH 6.8 Phosphate buffer-Prepare as directed in Buffer Solutions under Solutions in the section Reagents, Indicators, and Solutions. Mobile phase-Dissolve 0.5 g of sodium 1-decanesulfonate in 800 mL of water, add 50 mL of acetonitrile and 5 mL of glacial acetic acid, and mix. Adjust with 1 N sodium hydroxide to a pH of 4.4. Filter, and degas. Make adjustments if necessar y (see System Suitability under Chromatography <621>). Standard preparation-Quantitatively dissolve an accurately weighed quantity of USP Cycloserine RS in pH 6.8 Phosphate buffer to obtain a solution having a known concentration of about 0.4 mg per mL. Assay preparation-Transfer about 20 mg of Cycloserine, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with pH 6.8 Phosphate buffer to volume, and mix. Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 219-nm detector and a 4.6-mm × 25-cm column that contains 5- µm packing L1. The flow rate is about 1 mL per minute. The column temperature is maintained at about 30°. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 1.8; and the relative standard deviation for replicate injections is not more than 2.0%. Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the peak responses for cycloserine. Calculate the quantity, in µg, of C3H6N2O2 in each mg of Cycloserine taken by the formula: 50,000(C/W)(rU / rS) in which C is the concentration, in mg per mL, of USP Cycloserine RS in the Standard preparation; W is the quantity, in mg, of Cycloserine taken to prepare the Assay preparation; and rU and rS are the peak responses for cycloserine obtained from the Assay preparation and the Standard preparation, respectively.Production Process:
D-Cycloserine can be obtained through fermentation technique or through direct synthesis. The bacteria used in the fermentation is Actinomyces laven-dulae. The fermentation medium consists of dextrin, dextrose, starch, soybean powder, yeast powder, ammonium sulfate, ammonium nitrate, calcium carbonate, sodium chloride, magnesium sulfate and soybean oil. In the synthesis process, D-Cycloserine is obtained from β-Aminooxy alanine ethyl ester hydrochloride by reaction with potassium hydroxide in a cyclization reaction.