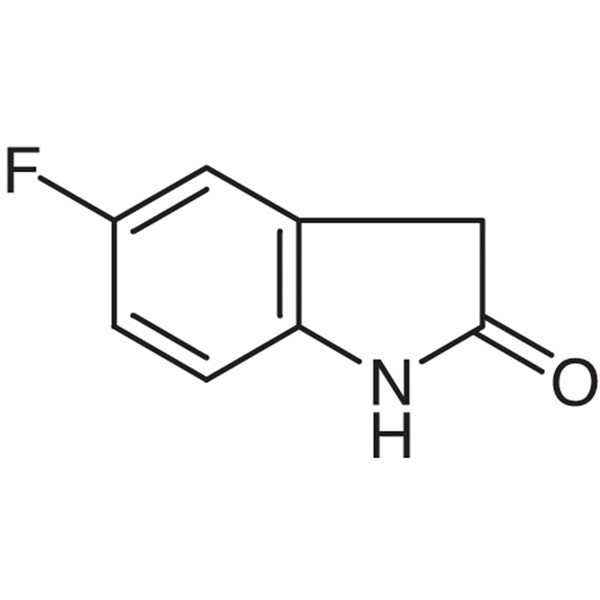
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 5-Fluoro-2-Oxindole (CAS: 56341-41-4) with high quality, commercial production. Welcomed to order.| Item | Specifications |
| Appearance | Light Yellow Crystalline Powder |
| 1 H NMR Spectrum | Consistent With Structure |
| LCMS | Consistent With Structure |
| Purity / Analysis Method | >99.0% (LCMS) |
| Melting Point | 143.0~147.0℃ |
| Loss on Drying | <0.50% |
| Total Impurities | <1.00% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates; Sunitinib Malate Intermediate |
Description:
Specifications:
Package & Storage:
| Chemical Name | 5-Fluoro-2-Oxindole |
| Synonyms | 5-Fluorooxindole; 5-Fluoro-2-indolinone; 5-Fluoroindolin-2-one; 5-Fluoro-1,3-Dihydro-indol-2-one |
| CAS Number | 56341-41-4 |
| CAT Number | RF-PI1543 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H6FNO |
| Molecular Weight | 151.14 |
| Density | 1.311±0.06 g/cm3 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
5-Fluoro-2-Oxindole (CAS: 56341-41-4) is mainly used in pharmaceutical industry as a pharmaceutical intermediate. 5-Fluoro-2-Oxindole is an intermediate in the synthesis of Sunitinib Malate (CAS: 341031-54-7). Sunitinib Malate is a kind of novel oral multi-targeted anticancer drugs and belongs to multi-targeted tyrosine kinase inhibitor with its trade name being “Suntent”. It was successfully developed by Pfizer Company and has dual anti-tumor effect. Moreover, it is the only therapeutic drug which can go beyond the 2-year survival period of advanced kidney cancer and plays a central role in the field of carcinoma and gastrointestinal stromal tumor therapeutic areas. It entered into market in February 2006 in the United States. This drug was the first anti-cancer drug which was approved by the US FDA and can simultaneously treat two diseases.