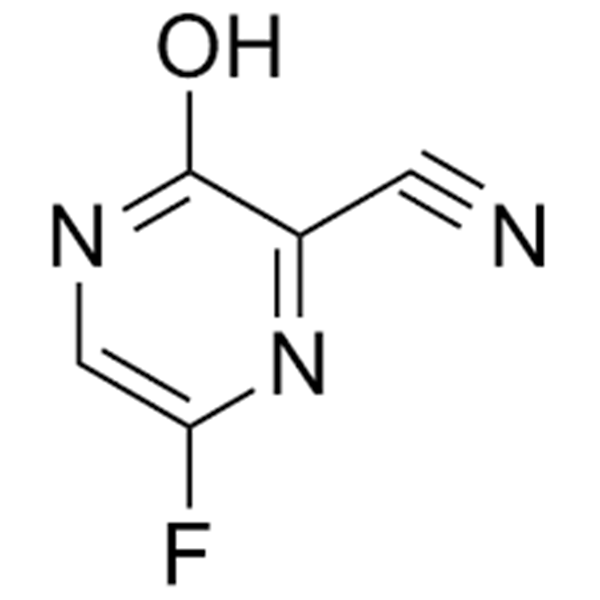
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer with High Purity and Stable Quality Commercial Supply Favipiravir and Related Intermediates: Favipiravir CAS 259793-96-9 2-Aminopropanediamide CAS 62009-47-6 Diethyl Aminomalonate Hydrochloride CAS 13433-00-6 3,6-Dichloropyrazine-2-Carbonitrile CAS 356783-16-9 3,6-Difluoropyrazine-2-Carbonitrile CAS 356783-28-3 6-Fluoro-3-Hydroxypyrazine-2-Carbonitrile CAS 356783-31-8 6-Bromo-3-Hydroxypyrazine-2-Carboxamide CAS 259793-88-9 3-Hydroxypyrazine-2-Carboxamide CAS 55321-99-8| Item | Specifications |
| Appearance | Pale Yellow to Off-White Powder |
| Identification | IR, HPLC |
| Moisture (K.F) | ≤1.0% |
| Residue on Ignition | ≤0.50% |
| Single Impurity | ≤1.0% |
| Total Impurities | ≤2.0% |
| Heavy Metals | ≤20ppm |
| Purity | ≥98.0% (by HPLC) |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Favipiravir (CAS 259793-96-9); Antiviral; COVID-19 |
Description:
Specifications:
Package & Storage:
| Chemical Name | 6-Fluoro-3-Hydroxypyrazine-2-Carbonitrile |
| Synonyms | 6-Fluoro-3-oxo-3,4-Dihydropyrazine-2-Carbonitrile |
| CAS Number | 356783-31-8 |
| CAT Number | RF-API294 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C5H2FN3O |
| Molecular Weight | 139.09 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
6-Fluoro-3-Hydroxypyrazine-2-Carbonitrile (CAS 356783-31-8) is an Intermediate of Favipiravir (CAS 259793-96-9). Favipiravir is a new broad-spectrum antiviral drug targeting RNA-dependent RNA polymerase (RdRp). Favipiravir was originally developed in the late 1990s by a company that was later purchased by the Japanese firm Fujifilm as part of its transition from the photo business to healthcare. After being tested against a range of viruses, the drug was approved in Japan in 2014 for emergency use against flu epidemics or to treat new strains of influenza. During the outbreak of the new coronavirus, the results of the Phase I clinical study of the drug published in March 2020 showed that the drug may have the effect of speeding up virus clearance to alleviate the progress of new coronavirus pneumonia. Favipiravir can be used in the treatment of patients with COVID-19 recommended by WHO. It was cleared by the Drugs Controller General of India (DCGI) for “emergency restricted” use among COVID-19 patients.