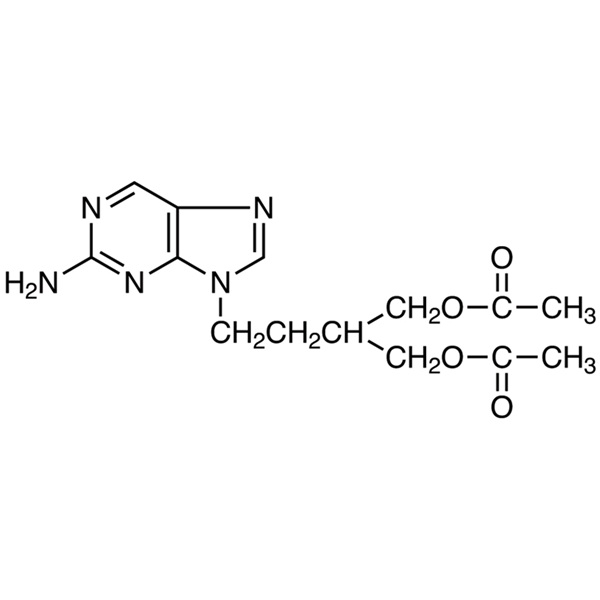
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Supply With High Purity, Commercial Production Chemical Name: Famciclovir CAS: 104227-87-4| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Identification | HPLC: Conforms to Standard |
| Identification | IR Absorption: Conforms to Standard |
| Melting Point | 102.0~104.0℃ |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.20% |
| Heavy Metals | ≤10ppm |
| Arsenic (As) | ≤1ppm |
| Test Standard | Enterprise Standard |
| Usage | API |
Description:
Specifications:
Package & Storage:
| Chemical Name | Famciclovir |
| Synonyms | BRL 42810; 2-[2-(2-Amino-9H-purin-9-yl)ethyl]-1,3-propanediol Diacetate |
| CAS Number | 104227-87-4 |
| CAT Number | RF-API104 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C14H19N5O4 |
| Molecular Weight | 321.34 |
| Solubility | Soluble in Methanol, Ethanol; Slightly Soluble in Water |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Famciclovir (CAS: 104227-87-4) is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections, most commonly for herpes zoster (shingles), treatment of herpes simplex virus 2 (genital herpes), herpes labialis (cold sores) in immunocompetent patients and for the suppression of recurring episodes of herpes simplex virus 2. It is also indicated for treatment of recurrent episodes of herpes simplex in HIV patients. It is a prodrug form of penciclovir with improved oral bioavailability. Famciclovir is marketed under the trade name Famvir (Novartis). Famciclovir was patented in 1983 and approved for medical use in 1994. In 2007, the United States Food and Drug Administration approved the first generic version of Famciclovir.