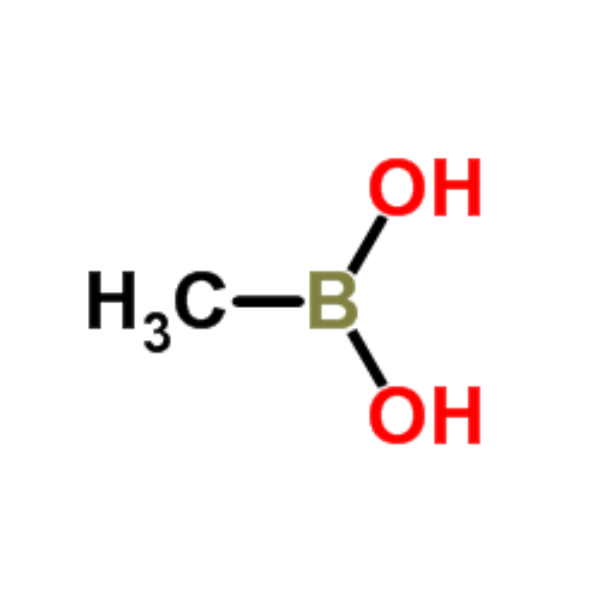
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply With High Quality, Commercial Production Chemical Name: Methylboronic Acid CAS: 13061-96-6| Item | Specifications |
| Appearance | White Crystals |
| Purity / Analysis Method | >98.0% |
| Melting Point | 87.0~94.0℃ |
| 1H NMR | Consistent With Structure |
| Infrared Spectrum | Conforms to Structure |
| Total Impurities | <2.00% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Description:
Specifications:
Package & Storage:
| Chemical Name | Methylboronic Acid |
| Synonyms | Methaneboronic Acid |
| CAS Number | 13061-96-6 |
| CAT Number | RF-PI1437 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | CH5BO2 |
| Molecular Weight | 59.86 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Methylboronic Acid (CAS: 13061-96-6) is a methylated derivative of boronic acid, a building block towards various intermediates in suzuki coupling, has many applications in organic synthesis. Methylboronic Acid can be used as a reagent: In the palladium-catalyzed Stille and Suzuki-Miyaura cross-couplings. In the microwave-heated heterogeneous palladium (Pd)-catalytized reactions. In ruthenium (Ru)-catalyzed silylation reactions To prepare bis(aminotropone) titanium (Ti) catalysts for ethylene polymerizations. In the enantioselective asymmetric bromoaminocyclization and bromoaminocyclization using amino-thiocarbamate catalysts. To prepare common building blocks for pharmaceuticals and agrochemicals. To prepare chrysin analogs by Suzuki-Miyaura coupling reactions. To prepare casein kinase I inhibitors. In the divergent C-H functionalizations directed by sulfonamide pharmacophores in drug discovery. In the synthesis of unsymmetrical monosulfides from disulfides via copper-catalyzed coupling with boronic acids. In a palladium-catalyzed coupling with enol tosylates. It is an important intermediate for the preparation of many boric acid derivatives such as (S) or (R) -2-Methyl-CBS-oxazaborolidine.