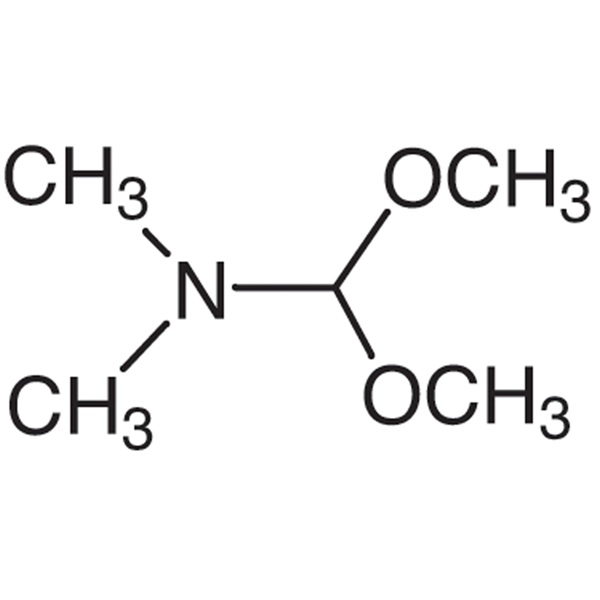
Chemical Properties:
Package: Bottle, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in a tightly closed container. Store in a cool, dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery. Shanghai Ruifu Chemical is the leading manufacturer of N,N-Dimethylformamide Dimethyl Acetal (DMF-DMA) (CAS: 4637-24-5) with high quality. Ruifu can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase DMF-DMA, Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | Colorless Transparent Liquid |
| Purity / Analysis Method | >99.0% (GC) |
| Total Impurities | ≤1.00% |
| Infrared Spectrum | Conforms to Structure |
| Attention | Avoid water, which can cause product purity to decrease |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Description:
Specifications:
Package & Storage:
| Chemical Name | N,N-Dimethylformamide Dimethyl Acetal |
| Synonyms | DMF-DMA; 1,1-Dimethoxytrimethylamine; 1,1-Dimethoxy-N,N-Dimethylmethylamin; N-Dimethoxymethyl-N,N-Dimethylamine |
| CAS Number | 4637-24-5 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C5H13NO2 |
| Molecular Weight | 119.16 |
| Boiling Point | 102.0~103.0℃/720 mmHg (lit.) |
| Specific gravity (20/20) | 0.8940 to 0.8980 |
| Refractive Index n20/D | 1.3950 to 1.3980 (lit.) |
| Sensitive | Moisture Sensitive |
| Solubility | Miscible With Most Organic Solvents |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
Advantages:
FAQ:

4637-24-5 - Risk and Safety:
Risk Codes R11 - Highly Flammable R22 - Harmful if swallowed R36/37/38 - Irritating to eyes, respiratory system and skin. R36/38 - Irritating to eyes and skin. R20 - Harmful by inhalation R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed. R10 - Flammable R52 - Harmful to aquatic organisms Safety Description S16 - Keep away from sources of ignition. S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 - Wear suitable protective clothing and gloves. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. S33 - Take precautionary measures against static discharges. S29 - Do not empty into drains. S7/9 - UN IDs UN 3271 3/PG 2 WGK Germany 1 FLUKA BRAND F CODES 21 TSCA Yes Hazard Class 3 Packing Group II Toxicity LD50 orally in Rabbit: > 5000 mg/kg4637-24-5 - Application:
N,N-Dimethylformamide Dimethyl Acetal (DMF-DMA) (CAS: 4637-24-5) has a wide range of applications, is an important intermediate in the synthesis of heterocyclic compounds, is also a good methylating reagent and vinegar dehydrating agent, especially as pharmaceutical synthesis intermediates. DMF-DMA has been used in methyl esterification of carboxylic acid. DMF-DMA is used as an intermediate in the formation of pyridine derivatives. DMF-DMA is utilized for the derivatization of primary sulfonamides and trifluoroacetic acid. It is also used in the preparation of formamidine derivatives. It is used as a reagent for n-dimethylaminomethylene and methyl esters. DMF-DMA is used to catalyze the coupling of epoxides with carbon dioxide to prepare cyclic carbonates. DMF-DMA is an intermediate of Zaleplon (CAS: 151319-34-5).4637-24-5 - Applications in Organic Synthesis:
Since Meenvin reported the preparation of DMF-DMA (N,N-Dimethylformamide Dimethyl Acetal) in 1956, DMF-DMA has become a reagent often used in organic synthesis. DMF-DMA is widely used in the construction of five or six membered heterocyclic rings in ring closure reaction. DMF-DMA has mild reaction and high yield, especially for high resistance compounds. The general structure of amide acetal compounds is as follows: The most widely used is DMF-DMA and DMF-DEA, amide acetal is easily hydrolyzed, can be esterified, amidine, alkylation and cyclization reactions. The central carbon atom of DMF-DMA is connected with three heteroatoms with large electronegative, which makes it have strong electrophilic activity. Under the action of acid, alkoxy group is easy to leave, and positive ions with stronger electrophilic activity are obtained. The reaction of DMF-DMA mainly consists of methylation reaction and formation reaction. "One-carbon Syntheson" of DMF-DMA In the loop closure reaction involving DMF-DMA, only one carbon atom in the product is often provided by DMF-DMA, so DMF-DMA can be regarded as a carbon syntheson. DMF-DMA esterification reaction DMF-DMA esterification enables various carboxylic acids to easily generate C1-20 alkyl or aryl esters, and byproducts can be easily separated by distillation. Adipic acid and DMF-DMA were esterified at 80 degrees for two hours. Amide acetal is an ideal choice for esterification of some carboxylic acids with steric hindrance or poor stability. Reaction of DMF-DMA amidination and protection of primary amines Amide acetals can react not only with primary amines, but also with amides, carbamates, sulfonamides to form hydrocarbon bonds. Such as: 2, 4-dimethyl aniline and DMF-DMA at 80 degrees can quickly remove methanol to form dimethamidine compounds. DMF-DMA can also be used as a primary amine protection group, primary amine protection group (2 N-H all protection) probably most people think of phthalyl, pyrrole ring, double Boc, double PMB, etc., but DMF-DMA protection of primary amine, in some cases is also very useful protection scheme, off protection only need TFA a mix. Amino protection - 13 common protection base introduction, protection base selection experience, range of use, introduction conditions, removal conditions summary sharing DMF-DMA reacts with active methyl and methylene groups to form carbon-carbon double bonds Phenylmethylation of DMF-DMA Heterocyclic compound reactions in DMF-DMA Amide acetal as a single carbon donor can be used to synthesize various complex compounds and biomimetic natural substances. With amide acetals can be synthesized: 1,2.4 triazole, 1.2, 4 triazolone, aminoheterocyclic derivatives, pyrimidine, pyrimidine, indoles, pyridine, quinoline, thiazole, oxazolone, isooxazole, 1.2, 4-triazone, pyranone, pyrazine, pyrazine and other series of ammonia heterocyclic derivatives, can also be synthesized oxygen, sulfur heterocyclic compounds. According to the type of chemical reaction, the application of amide acetal in heterocyclic compound synthesis can be divided into the following three aspects. (1): amide acetal and amine, amide, carbamate lipid reaction, generate a variety of heterocyclic rings Amidoacetal and amine reaction to formamidine intermediate, and then intramolecular nucleophilic ring reaction to generate various heterocycles, or formamidine and hydrazine, hydroxylamine, l, 2, one or two alkyl halides containing two active groups of compounds plus long carbon chain, and then intramolecular ring. Synthesis of heterocyclic compounds by the reaction of amide acetals and amides, such as the synthesis of l, 2,4 monotriazole derivatives. First, the acetal reacts with amide to form N, N 'tritradil, and then rings with phenylhydrazine to form l, 2,4 monotriazole derivatives Amide acetals react with carbamic acid or acetate to form chlorine-containing heterocyclic rings. A diactive intermediate formed by the reaction of an amide acetal with an aminoethyl ester: n.n-dimethyl-n 'alkyl-carboxymethyl formamidine, which reacts with hydrazine or substituted hydrazine. For example, for the preparation of 1,2,4 triazinone-6, the equation is shown below. If you react it with a nitro-formate you get 1,2,4 triazolone-5. Reaction mechanism for the formation of 1,2,4 triazolone-5 The composition of 1.2.4- triazolidin-5 is two steps. First, ethyl carbamate and DMF diformaldehyde acetals form the intermediate Nn-dimethyl-n-ethoxy-formamidine. Second, the amino group on phenylhydrazine deattacks the carbon on formamidine, which loses -N (CH3). Then the ammonia on the benzene ring near the phenylhydrazine attacks the carbon on the carbon group, forming an oxygen anion, and the lone pair of electrons on the oxygen comes down, loses the ethoxy group, and produces 1.2, 4-triazolone-5. (II) Preparation of heterocyclic compounds by the reaction of amide acetal and amide This is the most reported method of synthesis impurity in recent decades. The action of amide acetal is equivalent to Grignard reagent, but the reaction condition of amide acetal is simple and mild. Amide acetal has two active groups, high reactivity, and active methyl, methylene reaction to form amidine intermediates, can be further reaction, ring closure, and Grignard reagent and methylene reaction, only lengthening the carbon chain, can not be further reaction. For example, synthesis of furanochroone derivatives. (3): amide acetal and hydroxyl, sulfhydryl compound reaction to generate oxygen, sulfur heterocyclic compounds The above synthesis of furohutanone is a good example of the acetal generation of enamine derivatives and hydroxyl group by separating the endolateral pole, resulting in oxy-containing heteramine:. Another example: catechol and DMF -- DMA form oxygen-containing rings in the presence of dichloromethane. The reaction of DMF -- DMA and o-mercaptoaniline can produce sulfur-containing heterocyclic ring, the reaction formula is as follows Case study of DMF-DMA ring closure reaction and personal reaction (1) Batcho-Leimgruber indole synthesis Preparation of various Vindol derivatives from o-nitrotoluene. Reaction mechanism First of all, dimethylformamide dimethylacetal, the negative ions of the methoxy group leave to produce a more reactive intermediate. It is attacked by carboanions formed by the deprotonation of o-nitrotoluene methyl hydrogen and loses the methanol to obtain the above-mentioned enylamine. The product of this step, enamine, resembles an alkene with electron-withdrawing and electron-donating substituents attached on both sides (Push-pull olefin is strongly polar and often dark red because of the large conjugation range in the molecule. In the second step of the reaction, the nitro group is reduced to an amino group, followed by cyclization and elimination to obtain the final product. (2) Composite pictures of pyridine derivatives (3) Synthesis of pyrazole derivatives