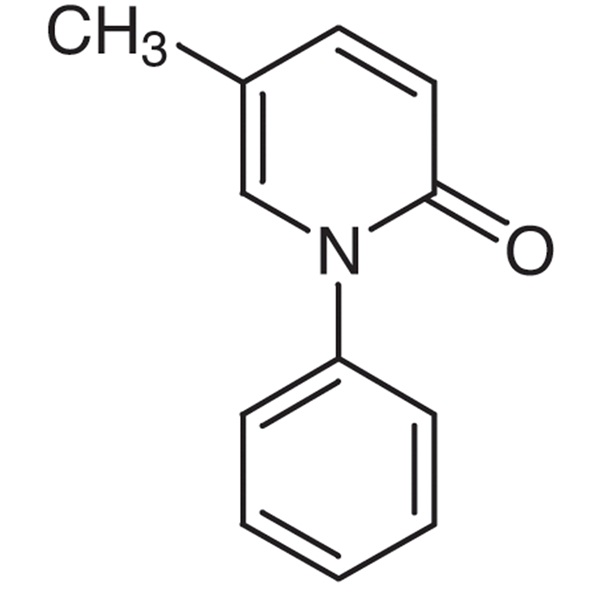
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Supply With High Purity, Commercial Production Chemical Name: Pirfenidone CAS: 53179-13-8| Item | Specifications |
| Appearance | White to Light Yellow Crystalline Powder |
| Identification | IR |
| Purity | ≥99.0% |
| Loss on Drying | ≤0.50% |
| Sulfated Ash | ≤0.20% |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Heavy Metals | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | API, Idiopathic Pulmonary Fibrosis (IPF) |
Description:
Specifications:
Package & Storage:
| Chemical Name | Pirfenidone |
| Synonyms | Perfenidone; 5-Methyl-1-Phenylpyridine-2(1H)-one; PFD; S-7701; AMR-69 |
| CAS Number | 53179-13-8 |
| CAT Number | RF-API108 |
| Stock Status | In Stock |
| Molecular Formula | C12H11NO |
| Molecular Weight | 185.22 |
| Melting Point | 96.0 to 97.0℃ |
| Solubility | Soluble in DMSO |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Pirfenidone (CAS: 53179-13-8) is in a group of medicines called antifibrotic agents. Pirfenidone has demonstrated activity in multiple fibrotic conditions, including those of the lung, kidney and liver. It affects your body’s immune system and reduces the amount of fibrosis (scarring) in the lungs. Pirfenidone is one of two medicines that are approved in Canada to treat Idiopathic Pulmonary Fibrosis (IPF). Pirfenidone is an inhibitor for TGF-β production and TGF-β stimulated collagen production, reduces production of TNF-α and IL-1β, and also has anti-fibrotic and anti-inflammatory properties. It was first approved in Japan for the treatment of patients with idiopathic pulmonary fibrosis after clinical trials, under the trade name of Pirespa by Shionogi, in 2008. It was approved for use in the European Union in 2011, in Canada in 2012, and in the United States in October 2014.