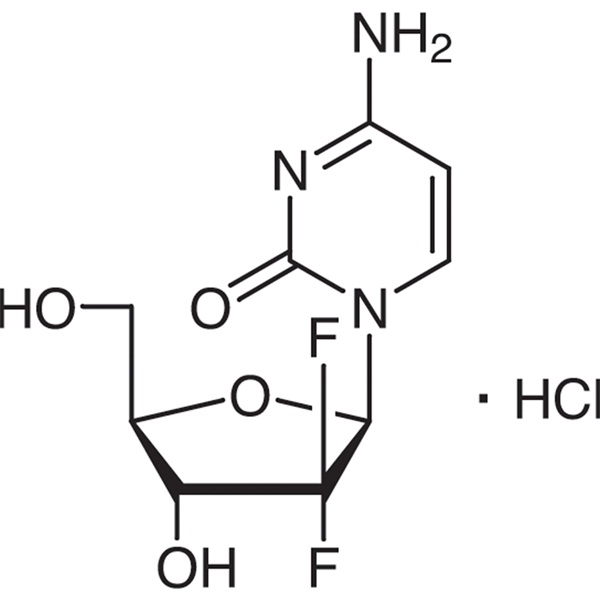
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer with High Purity and Stable Quality Chemical Name: Gemcitabine Hydrochloride CAS: 122111-03-9 An Anticancer Agent API USP Standard, High Quality, Commercial Production| Item | Specifications |
| Appearance | White Crystal Powder, Odorless |
| Solubility | Soluble in Water, slightly soluble in methanol, practically insoluble in acetone |
| Identifiaction IR | IR spectrum should be concordant to that of the reference standard |
| Identifiaction Chloride | Positive. It meets the requirements of the tests for chloride |
| Appearance of Solution | Solution S in clear and not more intensely colored than reference solution BY7 |
| pH | 2.0~3.0 |
| Specific Rotation | +43.0° ~ +50.0° |
| Heavy Metals (Pb) | ≤10ppm |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.10% |
| Related Substances | |
| Cytosine | ≤0.10% |
| α-Isomer | ≤0.10% |
| Any Other Impurity | ≤0.10% |
| Total Impurities | ≤0.20% |
| Residual Solvents | |
| Methanol | ≤0.30% |
| Toluene | ≤0.01% |
| Dichloromethane | ≤0.01% |
| Acetone | ≤0.50% |
| Assay | 98.0%~102.0% (Calculated on dried base) |
| Test Standard | United States Pharmacopeia (USP) Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Description:
Specifications:
Package & Storage:
| Chemical Name | Gemcitabine Hydrochloride |
| Synonyms | Gemcitabine HCl; 2'-Deoxy-2',2'-difluorocytidine; dFdC |
| CAS Number | 122111-03-9 |
| CAT Number | RF-API41 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C9H12ClF2N3O4 |
| Molecular Weight | 299.66 |
| Melting Point | >250℃ |
| Shipping Condition | Under Ambient Temperature |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Gemcitabine Hydrochloride (CAS: 122111-03-9) with high quality API. Gemcitabine Hydrochloride (CAS: 122111-03-9) is a synthetic novel difluoro nucleoside drug that is anti-metabolic and antineoplastic. It is researched and developed by the Eli Lilly and Company and approved to be listed in South Africa, Sweden, the Netherlands, Australia and other countries in 1995. The United States Food and Drug Administration (FDA) approved it as the first-line therapy for the clinical treatment of non-small cell lung cancer and pancreatic cancer. Gemcitabine hydrochloride, as a pro-drug, is a good substrate for the acidification of deoxygenation of thymine kinase phosphorus in the cell, and under the action of the enzyme, it can be converted into the following metabolites: gemcitabine mono-phosphate (dFdCMP), gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP), among which the latter two are the active products.