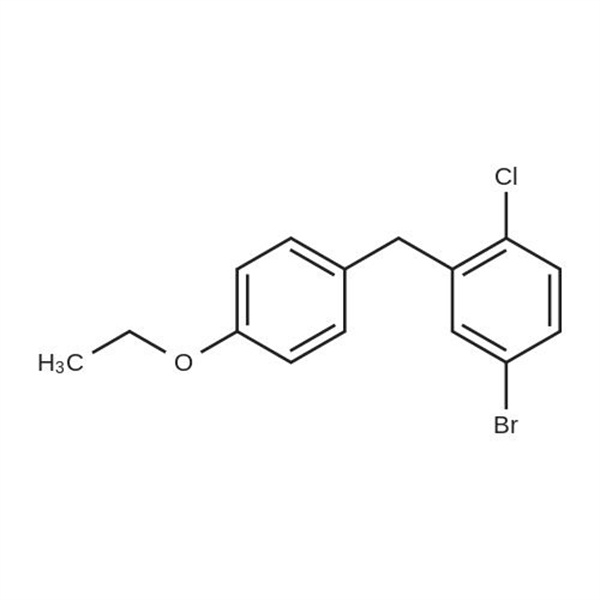
Chemical Properties:
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply, High Purity, Commercial Production Dapagliflozin (CAS: 461432-26-8) Related Intermediates: 5-Bromo-2-Chlorobenzoic Acid CAS 21739-92-4 5-Bromo-2-Chloro-4'-Ethoxydiphenylmethane CAS 461432-23-5 2,3,4,6-Tetrakis-O-Trimethylsilyl-D-Gluconolactone CAS 32384-65-9| Item | Specifications |
| Appearance | White to Pael Yellow Crystalline Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Water (K.F) | ≤0.50% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Dapagliflozin (CAS: 461432-26-8), type II diabetes |
Description:
Specifications:
Package & Storage:
| Chemical Name | 5-Bromo-2-Chloro-4'-Ethoxydiphenylmethane |
| Synonyms | 4-Bromo-1-Chloro-2-(4-Ethoxybenzyl)benzene; 4-(5-Brom-2-Chlorbenzyl)phenyl-Ethylether; Dapagliflozin Bromo Impurity |
| CAS Number | 461432-23-5 |
| CAT Number | RF-PI447 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C15H14BrClO |
| Molecular Weight | 325.63 |
| Melting Point | 41.0 to 43.0℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
4-Bromo-1-Chloro-2-(4-Ethoxybenzyl)benzene (CAS: 461432-23-5) is used as a intermediate in the synthesis of Dapagliflozin (CAS: 461432-26-8). Dapagliflozin is a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Dapagliflozin is a new antidiabetic drug jointly developed by Bristol-Myers Squibb and AstraZeneca, being approved by the European Medicines Agency (EMA) on November 12, 2012. On January 8, 2014, the US Food and Drug Administration (FDA) have approved it for being used in the treatment of type II diabetes.