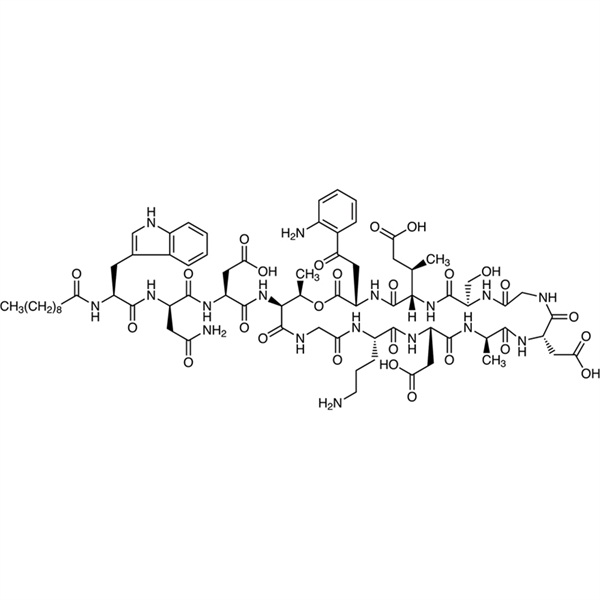
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer with High Purity and Stable Quality Chemical Name: Daptomycin CAS: 103060-53-3 API High Quality, Commercial Production| Item | Specifications |
| Appearance | Yellow or Pale Yellow Powder |
| Identification HPLC | The retention time of major peak of the sample solution should correspond to that of reference standard. |
| Identification IR | IR spectrum of the test specimen should consistent with the IR spectrum of reference standard. |
| Appearance of Solution | Clarity The solution should be clear or not more pronounced than that of reference suspension II. |
| Specific Optical Rotation | +17.0° to +25.0° |
| pH | 4.0 to 5.0 |
| Residue on Ignition | ≤1.0% |
| Anhydro-Daptomycin | ≤2.5% |
| β-Isomer | ≤0.50% |
| Hydrolysis Impurity | ≤0.50% |
| Impurity 1 | ≤0.75% |
| Impurtiy 2 | ≤0.75% |
| Impurtiy 3 | ≤0.75% |
| Any Other Impurity | ≤0.15% |
| Total Impurities | ≤5.0% |
| Heavy Metals | ≤30ppm |
| Water | ≤5.0% |
| Purity | ≥95.0% (Calculated on the dried basis) |
| Bacterial Endotoxins | <0.3EU/mg |
| Residual Solvents n-Butanol | ≤5000ppm |
| ResidualSolvents Isopropanol | ≤5000ppm |
| Residual Solvents Ethanol | ≤5000ppm |
| Microbial Limit TAMC | ≤100cfu/g |
| Microbial Limit TYMC | ≤10cfu/g |
| E.Coil | Not Detected |
| Test Standard | Enterprise Standard |
| Storage Conditions | Preserve in tight containers, and store at -25~-10℃. |
| Usage | Active Pharmaceutical Ingredient (API) |
Description:
Specifications:
Package & Storage:
| Chemical Name | Daptomycin |
| Synonyms | LY146032 |
| CAS Number | 103060-53-3 |
| CAT Number | RF-API10 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C72H101N17O26 |
| Molecular Weight | 1620.69 |
| Melting Point | 202.0~204.0℃ |
| Solubility | Solube in Methanol |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Daptomycin (CAS: 103060-53-3) is a kind of cyclic lipopeptide antibiotics with novel structure. It is extracted from the Streptomyces fermentation broth. It was discovered by Eli Lilly Company in the 1980s, and successfully developed in 1997 by Cubist Pharmaceuticals. It not only having a novel chemical structure but also has mode of action which is different from any antibiotic approved before: it inhibits cell by disrupting the transport of amino acids through cell membrane, thereby blocking the cell wall peptidoglycan biosynthesis and changing the nature of the cell membrane. It can destroy the bacterial cell membrane function in many aspects, and quickly kill gram-positive bacteria. In addition to the role of taking effect on most clinically relevant gram-positive bacteria, more importantly, Daptomycin has a potent efficacy in treating isolated strains which have shown signs of resistance to methicillin, vancomycin and linezolid. This property is of great clinical significance to patients suffering from severe infection. In September 2003, the US Food and Drug Administration had approved for the first time that Daptomycin (CAS: 103060-53-3) could be applied for the treatment of severe skin infections. In March 2006, it was approved for treating infectious diseases. In January 2006, it is approved by European Commission for the treatment of certain complicated skin and soft tissue infections caused by gram-positive bacteria. On September 6, 2007, Cubist Pharmaceuticals announced that the European Union has approved its antibacterial drug, Cubicin for the treatment of right heart endocarditis caused by Staphylococcus aureus infections and complicated skin and soft tissue infection related diseases caused by Staphylococcus aureus.