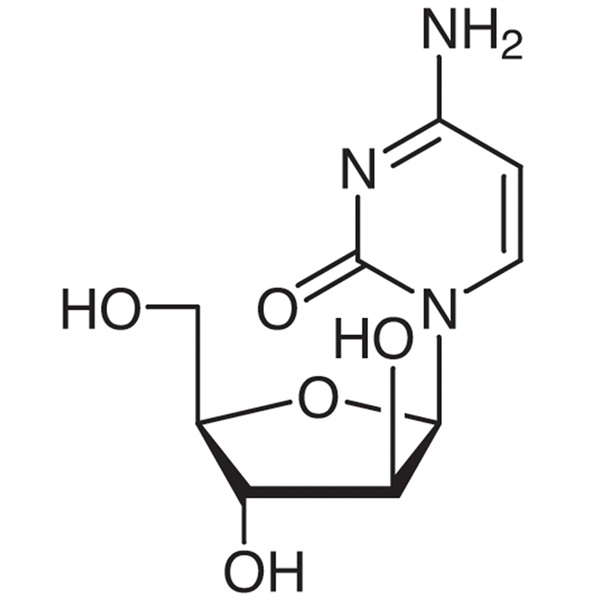
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply Arabinonucleosides Intermediates with High Purity Vidarabine; Ara-A; CAS: 5536-17-4 Arabinofuranosyluracil; Ara-U ; CAS: 3083-77-0 Cytarabine; Ara-C; CAS: 147-94-4| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| Specific Rotation | +154°~+160° |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.50% |
| Heavy Metals | ≤10ppm |
| Total Impurities | ≤0.30% (HPLC) |
| Uridine | ≤0.10% (HPLC) |
| Uracil | ≤0.10% (HPLC) |
| Arabinofuranosyluracil | ≤0.30% (HPLC) |
| Assay | 98.0% ~102.0% |
| Test Standard | United States Pharmacopeia (USP) |
| Usage | API; Pharmaceutical Intermediates |
Description:
Specifications:
Package & Storage:
| Chemical Name | Cytarabine |
| Synonyms | Ara-C; Arabinocytidine; Cytosine β-D-Arabinofuranoside; Arabinofuranosylcytosine |
| CAS Number | 147-94-4 |
| CAT Number | RF-PI218 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C9H13N3O5 |
| Molecular Weight | 243.22 |
| Melting Point | 214℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Cytarabine (CAS: 147-94-4) is a kind of purine nucleoside-class antiviral chemical synthesis that initially extracted from the medium of streptomyces, and then produced from chemical synthesis. It is a white crystalline powder and is very slightly soluble in water. Its monophosphate ester is easily soluble in water. It has inhibitory effect on various kinds of DNA virus such as Herpes simplex virus HSV1 and HSV2, hepatitis B virus, varicella-zoster virus and cytomegalovirus. Cytarabine (CAS: 147-94-4), a chemotherapy agent used mainly in the treatment of cancers of white blood cells such as acute myeloid leukemia (AML) and non-Hodgkin lymphoma . Cytarabine is a cytosine analogue and antineoplastic agent used largely in the therapy of acute leukemia. Cytarabine is associated with a low rate of transient serum enzyme and bilirubin elevations during therapy, but has only rarely been implicated in cases of clinically apparent acute liver injury with jaundice.