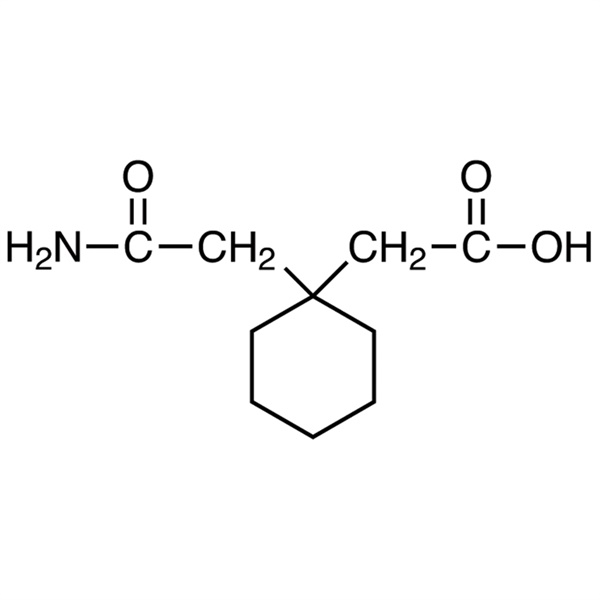
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply Gabapentin Related Intermediates: Gabapentin CAS 60142-96-3 1,1-Cyclohexanediacetic Acid (CDA) CAS 4355-11-7 Gabapentin-Lactam (CDI) CAS 64744-50-9 1,1-Cyclohexanediacetic Anhydride (CAA) CAS 1010-26-0 3,3-Pentamethylene Glutarimide (CAI) CAS 1130-32-1 1,1-Cyclohexanediacetic Acid Monoamide (CAM) CAS 99189-60-3| Item | Specifications |
| Appearance | White Crystalline Powder |
| Purity / Analysis Method | >99.0% (HPLC) |
| Melting Point | 144.0~148.0℃ |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Imine | <1.0% |
| Ammonium Salt | <500ppm |
| CDA | <1.00% (1,1-Cyclohexanediacetic Acid, CAS: 4355-11-7) |
| Total Impurities | <1.00% |
| Heavy Metals | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Gabapentin (CAS: 60142-96-3) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 1,1-Cyclohexanediacetic Acid Monoamide |
| Synonyms | CAM; 1-(Carbamoylmethyl)cyclohexaneacetic Acid; 1-(2-Amino-2-oxoethyl)cyclohexaneacetic Acid |
| CAS Number | 99189-60-3 |
| CAT Number | RF-PI1240 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H17NO3 |
| Molecular Weight | 199.25 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
1,1-Cyclohexanediacetic Acid Monoamide (CAM) (CAS: 99189-60-3) is a key intermediate of Gabapentin (CAS: 60142-96-3). Gabapentin is an anticonvulsant medication primarily used to treat partial seizures and neuropathic pain. It is a first-line medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. Gabapentin was first approved for use in 1993. It has been available as a generic medication in the United States since 2004. Gabapentin is an Amino acid structurally related to γ-Aminobutyric Acid (GABA), designed to cross the blood brain barrier.