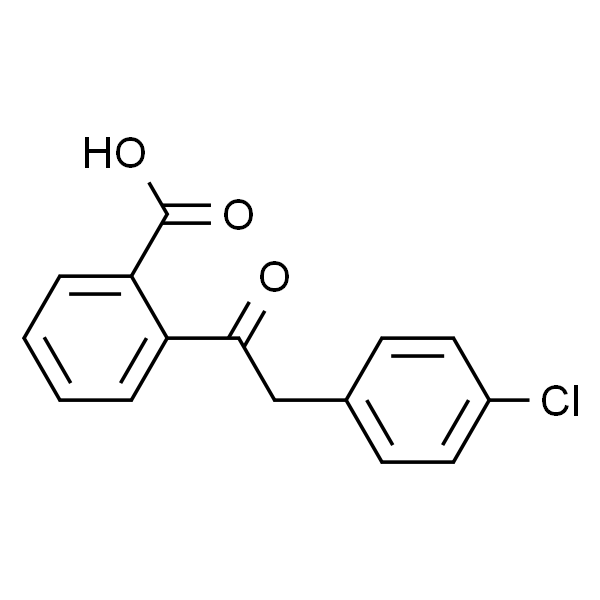
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply, High Purity, Commercial Production Chemical Name: 2-((4-Chlorophenyl)acetyl)benzoic Acid CAS: 53242-76-5 Intermediate of Azelastine Hydrochloride (CAS: 79307-93-0)| Item | Specifications |
| Appearance | White to Slight White Powder |
| Assay | 98.0~102.0% |
| Melting Point | 139.0 to 141.0℃ |
| Lossing on Drying | ≤0.50% |
| Total Impurities | ≤1.0% |
| Related Substances Single | |
| 4-Chloro Phenyl Acetic Acid | ≤0.20% |
| 1,2-Benzene Dicarboxylic Acid | ≤0.20% |
| Benzofuran | ≤0.20% |
| Single Unknown Impurities | ≤0.50% |
| Residual Solvents | |
| Toluene | ≤890ppm |
| Ethanol | ≤5000ppm |
| Benzene | ≤50ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Azelastine Hydrochloride (CAS: 79307-93-0) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 2-((4-Chlorophenyl)acetyl)benzoic Acid |
| Synonyms | 2-[(4-Chlorophenyl)acetyl]benzoic Acid; 2-[2-(4-Chlorophenyl)acetyl]benzoic Acid; Azelastine EP Impurity C |
| CAS Number | 53242-76-5 |
| CAT Number | RF-PI454 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C15H11ClO3 |
| Molecular Weight | 274.7 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
2-((4-Chlorophenyl)acetyl)benzoic Acid (CAS 53242-76-5) is an intermediate of Azelastine Hydrochloride (CAS: 79307-93-0). Azelastine hydrochloridem, is an orally effective antihistamine, is a potent, second-generation, and selective histamine 1 (H1) receptor antagonist. Azelastine hydrochloride can be used for the research of allergic rhinitis, asthma, diabetic hyperlipidemic and SARS-CoV-2. Azelastine, sold under the brand name Optivar among others, is a medication primarily used as a nasal spray to treat allergic rhinitis (hay fever) and as eye drops for allergic conjunctivitis. Other uses may include asthma and skin rashes for which it is taken by mouth. Onset of effects is within minutes when used in the eyes and within an hour when used in the nose. Azelastine was patented in 1971 and came into medical use in 1986. It is available as a generic medication.