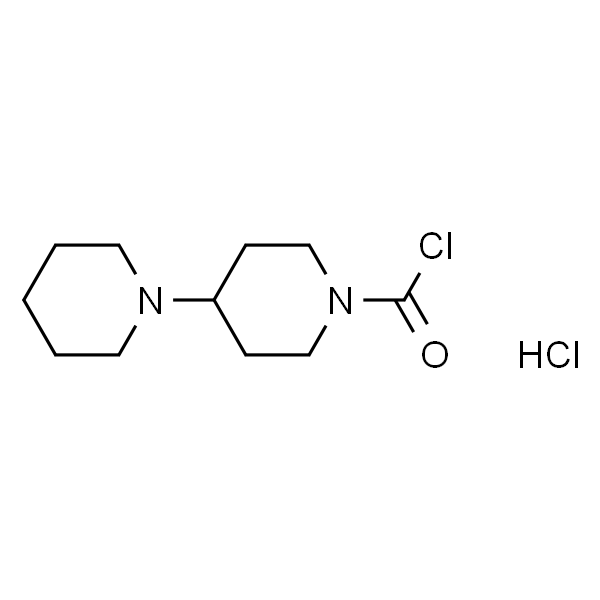
Chemical Properties:
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Manufacturer Supply Irinotecan and Related Intermediates: Irinotecan Hydrochloride CAS: 100286-90-6 Irinotecan Free Base CAS: 97682-44-5 Irinotecan Hydrochloride Trihydrate CAS: 136572-09-3 7-Ethyl-10-Hydroxycamptothecin CAS: 86639-52-3 1-Chlorocarbonyl-4-Piperidinopiperidine Hydrochloride CAS: 143254-82-4| Item | Specifications |
| Appearance | White or Light Yellow Powder |
| Assay | ≥98.0% (HPLC) |
| Loss on Drying | ≤1.0% |
| Heavy Metals | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Irinotecan Hydrochloride (CAS: 100286-90-6) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 1-Chlorocarbonyl-4-Piperidinopiperidine Hydrochloride |
| CAS Number | 143254-82-4 |
| CAT Number | RF-PI243 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C11H20Cl2N2O |
| Molecular Weight | 267.2 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 1-Chlorocarbonyl-4-Piperidinopiperidine Hydrochloride (CAS: 143254-82-4) with high quality. It is an intermediate typically in the synthesis of Irinotecan Hydrochloride (CAS: 100286-90-6), a DNA Topoisomerase I Inhibitor. lrinotecan hydrochloride, a semi-synthetic, water soluble derivative of the potent anticancer agent camptothecin, was launched in Japan for the treatment of lung, ovarian, and cervical cancers. lrinotecan exerts its antitumor activity via inhibition of topoisomerase I, a cellular enzyme that is involved in maintaining the topographic structure of DNA during the process of translation, transcription, and mitosis. lrinotecan undergoes de-esterification in vivo to yield an active metabolite, SN-38, which is 1000-fold more potent than the parent. Although being much less toxic than camptothecin, a significant number of patients in clinical trials exhibited side effects of leukopenia, diarrhea, nauseahromiting, and alopecia.