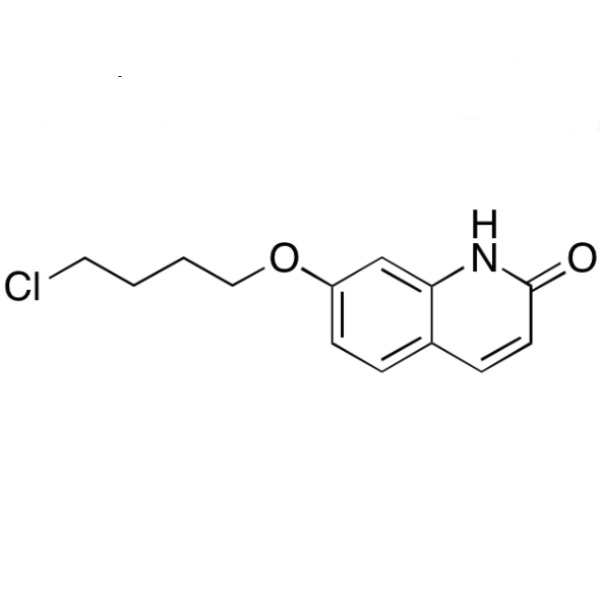
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureRuifu Chemical Supply Brexpiprazole Intermediates Brexpiprazole CAS 913611-97-9 7-Hydroxyquinolinone CAS 70500-72-0 4-Bromobenzo[b]thiophene CAS 5118-13-8 1-(1-Benzothiophen-4-yl)piperazine Hydrochloride CAS 913614-18-3 7-(4-Chlorobutoxy)quinolin-2(1H)-one CAS 913613-82-8 4-Chlorobenzo[b]thiophene CAS 66490-33-3| Item | Specifications |
| Appearance | Pale Yellow to Pale Beige Solid |
| Purity / Analysis Method | >98.0% (HPLC) |
| Infrared Spectrum | Conforms to Structure |
| NMR | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate / Impurity of Brexpiprazole (CAS: 913611-97-9) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 7-(4-Chlorobutoxy)quinolin-2(1H)-one |
| Synonyms | 7-(4-Chlorobutoxy)-1H-Quinolin-2-one; Brexpiprazole Impurity 23 |
| CAS Number | 913613-82-8 |
| CAT Number | RF-PI1980 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C13H14ClNO2 |
| Molecular Weight | 251.71 |
| Boiling Point | 460.8±45.0℃ |
| Density | 1.216±0.06 g/cm3 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
7-(4-Chlorobutoxy)quinolin-2(1H)-one (CAS: 913613-82-8) is an intermediate / impurity of Brexpiprazole (CAS: 913611-97-9). Brexpiprazole is an atypical antipsychotic drug. It is a D2 dopamine partial agonist called serotonin-dopamine activity modulator (SDAM). The drug received FDA approval on July 13, 2015 for the treatment of schizophrenia, and as an adjunctive treatment for depression. Although Brexpiprazole failed Phase II clinical trials for ADHD, Brexpiprazole has been designed to provide improved efficacy and tolerability (e.g., less akathisia, restlessness and/or insomnia) over established adjunctive treatments for major depressive disorder (MDD).