Chemical Properties:
Package: Bottle, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Commercial Supply Ensitrelvir (S-217622) Intermediates: S-Ethylisothiourea Hydrobromide CAS 1071-37-0 2,4,5-Trifluorobenzyl Bromide CAS 157911-56-3 Trimethyloxonium Tetrafluoroborate CAS 420-37-1 6-Chloro-2-methyl-2H-indazol-5-amine CAS 1893125-36-4 3-(Chloromethyl)-1-methyl-1H-1,2,4-triazole hydrochloride CAS 135206-76-7 1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)- CAS 1360105-53-8| Item | Specifications |
| Appearance | Light Brown to Gray Solid Powder |
| Purity / Analysis Method | >98.0% (LCMS) |
| 1 H NMR Spectrum | Consistent With Structure |
| LCMS | Consistent With Structure |
| Loss on Drying | ≤1.00% |
| Total Impurities | <2.00% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Ensitrelvir (S-217622), COVID-19 |
Description:
Specifications:
Package & Storage:
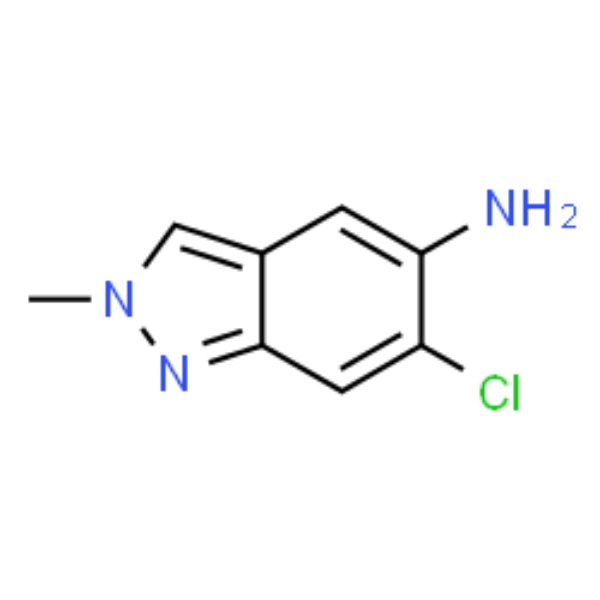
| Chemical Name | 6-Chloro-2-methyl-2H-indazol-5-amine |
| CAS Number | 1893125-36-4 |
| CAT Number | RF-PI1506 |
| Stock Status | In Stock |
| Molecular Formula | C8H8ClN3 |
| Molecular Weight | 181.62 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
6-Chloro-2-methyl-2H-indazol-5-amine (CAS: 1893125-36-4) is an intermediate of Ensitrelvir (S-217622). Ensitrelvir by Shionogi & Co. is a highly effective antiviral drug that works just as well as Pfizer's Paxlovid. Oral Xocova (Ensitrelvir), which has the same mechanism of action as Pfizer’s Paxlovid (Nirmatrelvir+Ritonavir), differs in that it only needs to be taken once a day, while the therapeutic effectiveness of ensitrelvir does not seem to be inferior to Paxlovid. As a reminder, Nirmatrelvir is administered twice daily, together with the booster represented by Ritonavir. Ensitrelvir became the first Japanese domestic pill to treat COVID-19, third to be regulatorally approved in Japan; in February 2022.