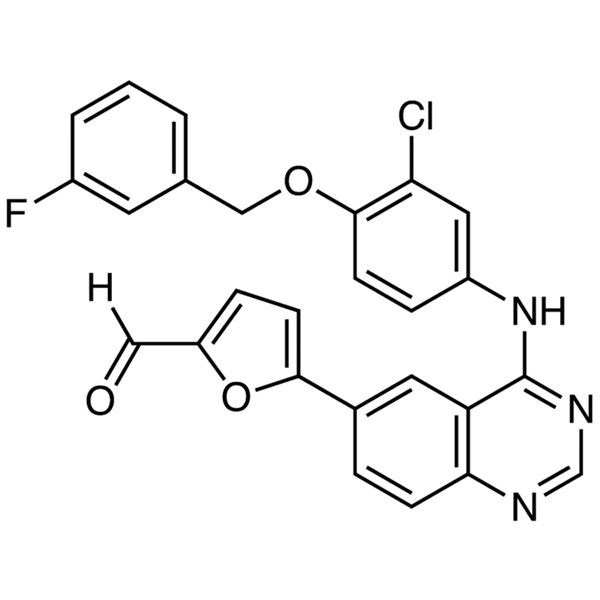
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureManufacturer Supply Related Intermediates: Sodium Methanesulfinate CAS 20277-69-4 2-Aminoethyl Methyl Sulfone Hydrochloride CAS 104458-24-4 6-Iodo-4-Hydroxyquinazoline CAS 16064-08-7 5-[4-[3-Chloro-4-(3-Fluorobenzyloxy)anilino]-6-Quinazolinyl]furan-2-Carboxaldehyde CAS 231278-84-5 CAS 231277-92-2 CAS 388082-77-7| Item | Specifications |
| Appearance | Pale Yellow to Yellow Solid Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Melting Point | 225.0~235.0℃ |
| Loss on Drying | <1.00% |
| Residue on Ignition | <0.50% |
| Total Impurities | <2.00% |
| Heavy Metals (as Pb) | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of API (CAS 388082-77-7) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 5-[4-[3-Chloro-4-(3-Fluorobenzyloxy)anilino]-6-Quinazolinyl]furan-2-Carboxaldehyde |
| Synonyms | 5-[4-[[3-Chloro-4-[(3-Fluorophenyl)methoxy]phenyl]amino]-6-Quinazolinyl]-2-Furancarboxaldehyde; 5-[4-((3-Chloro-4-((3-Fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-Furaldehyde |
| CAS Number | 231278-84-5 |
| CAT Number | RF-PI1500 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C26H17ClFN3O3 |
| Molecular Weight | 473.90 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
5-[4-[3-Chloro-4-(3-Fluorobenzyloxy)anilino]-6-Quinazolinyl]furan-2-Carboxaldehyde (CAS: 231278-84-5) is an intermediate of API (CAS 388082-77-7). (CAS 388082-77-7) is a drug targeting breast cancer developed by British GlaxoSmithKline Co. It is a tyrosine kinase inhibitor which can effectively inhibit the tyrosine kinase activity of human epidermal growth factor receptors 1 and 2 (ErbB1, ErbB2). It can uniquely act in a variety of ways, ensuring that breast cancer cells cannot receive growth signals. It inhibits intracellular EGFR (ErbB-1) and HER2 (ErbB-2) ATP sites, preventing tumor cell phosphorylation and activation, blocking down-regulation signals through the homogeneity and heterogeneity of EGFR (ErbB-1) and HER2 (ErbB-1) dimerization.