Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureRuifu Chemical Supply Candesartan Cilexetil Intermediate With High Purity Candesartan Cilexetil CAS 145040-37-5 Candesartan CAS 139481-59-7 1-Chloroethyl Cyclohexyl Carbonate CAS 99464-83-2 Candesartan Cilexetil Intermediate Eethyl Ester C6 CAS 139481-41-7 Candesartan Cilexetil Intermediate CAS 139481-44-0 Trityl Candesartan Cilexetil CAS 170791-09-0| Item | Specifications |
| Appearance | White Powder |
| Identification-HPLC | The Retention Time Similar to Standard |
| Identification-IR | Similar to Standard |
| Purity / Analysis Method | >99.0% (HPLC) |
| Melting Point | 183.0~185.0℃ |
| Loss on Drying | <0.50% |
| Moisture (K.F) | <0.50% |
| Residue on Ignition | <0.50% |
| Heavy Metals | <20ppm |
| Individual Impurity | <1.00% (HPLC) |
| Total Impurities | <1.00% (HPLC) |
| Refractive Index | n20/D 1.745~1.747 |
| Test Standard | Enterprise Standard |
| Usage | API; For the Treatment of Hypertension |
Description:
Specifications:
Package & Storage:
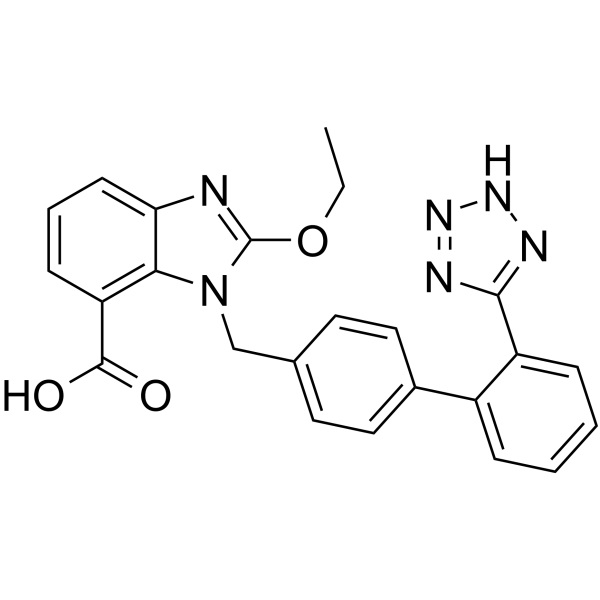
| Chemical Name | Candesartan |
| Synonyms | CV-11974; 2-Ethoxy-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-Benzimidazole-7-Carboxylic Acid; 3-[[2'-(1H-Tetrazol-5-yl)biphenyl-4-yl]methyl]-2-ethoxy-3H-Benzimidazole-4-Carboxylic Acid; Candesartan M1; Candesartan Cilexetil EP Impurity G |
| CAS Number | 139481-59-7 |
| CAT Number | RF-PI1889 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C24H20N6O3 |
| Molecular Weight | 440.45 |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Candesartan (CAS: 139481-59-7) is an angiotensin II receptor antagonist with IC50 of 0.26 nM. Target: Angiotensin II Receptor candesartan is indicated for the treatment of hypertension. Candesartan Cilexetil was first approved in GB on Apr 29, 1997, then approved by the U.S. Food and Drug Administration (FDA) on June 4, 1998, and approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on Mar 12, 1999. It was developed by AstraZeneca, then marketed as Atacand by AstraZeneca in GB and the US, and marketed as Blopress by Takeda in JP. Candesartan Cilexetil is an angiotensin II receptor blocker (ARB), it blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Atacand is indicated for the treatment of hypertension in adults and children 1 to < 17 years of age, heart failure (NYHA class II-IV) and used to reduce cardiovascular death and heart failure hospitalization.