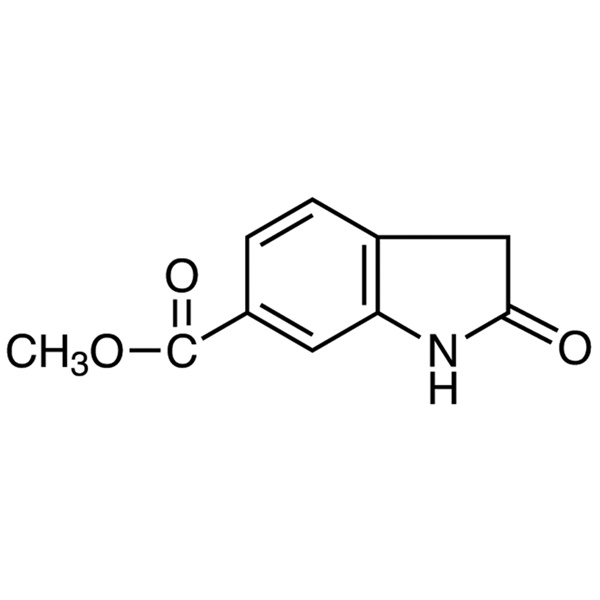
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Methyl 2-Oxoindoline-6-Carboxylate (CAS: 14192-26-8) with high quality, commercial production.| Item | Specifications |
| Appearance | Light Yellow to Brown Powder |
| 1 H NMR Spectrum | Consistent With Structure |
| Purity / Analysis Method | >99.0% (HPLC) |
| Loss on Drying | <1.00% |
| Total Impurities | <1.00% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Nintedanib Esylate (CAS: 656247-18-6) |
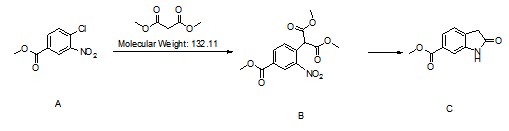
Description:
Specifications:
Package & Storage:
| Chemical Name | Methyl 2-Oxoindoline-6-Carboxylate |
| Synonyms | 2-Oxoindoline-6-Carboxylic Acid Methyl Ester; Methyl Oxindole-6-Carboxylate |
| CAS Number | 14192-26-8 |
| CAT Number | RF-PI1524 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H9NO3 |
| Molecular Weight | 191.19 |
| Melting Point | 184.0~190.0℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Methyl 2-Oxoindoline-6-Carboxylate (CAS: 14192-26-8) is an intermediate used to prepare Nintedanib Esylate (CAS: 656247-18-6). Nintedanib Esylate is a potent, oral triple angiokinase inhibitor developed by Boehringer Ingelheim that targets proangiogenic and pro-fibrotic pathways mediated by the vascular endothelial growth factor receptor, fibroblast growth factor receptor and plateletderived growth factor receptor families, as well as Src and Flt-3 kinases. Nintedanib Esylate was approved for the treatment of idiopathic pulmonary fibrosis (IPF), a condition in which the lungs become progressively scarred over time, by the US FDA in October 2014 and by the EMA in January 2015. The FDA granted nintedanib esylate fast-track, priority review, orphan product, and breakthrough designations. It was also approved by the EMA in November 2014 for treatment of non-small cell lung cancer in combination with docetaxel after first-line chemotherapy.