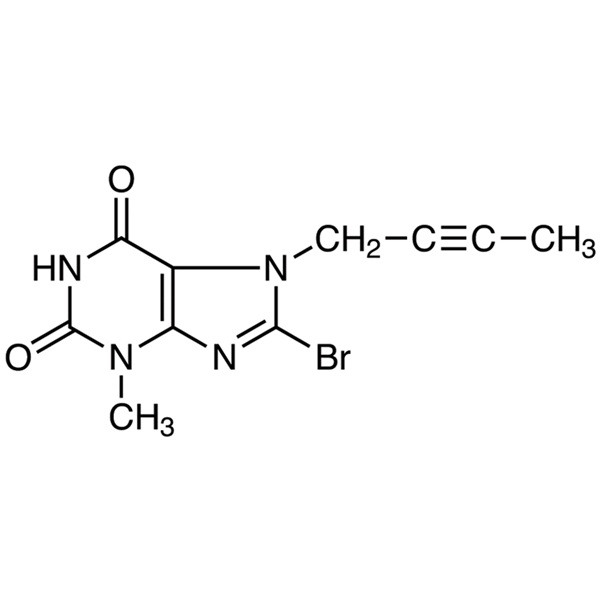
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.Manufacturer Supply Linagliptin and Related Intermediates: Linagliptin CAS 668270-12-0 Linagliptin Parent Nucleus Intermediate CAS 853029-57-9 8-Bromo-3-Methylxanthine CAS 93703-24-3 8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine CAS 666816-98-4 2-(Chloromethyl)-4-Methylquinazoline CAS 109113-72-6 (R)-3-(Boc-Amino)piperidine CAS 309956-78-3 (R)-(-)-3-Aminopiperidine Dihydrochloride CAS 334618-23-4 1-Bromo-2-Butyne CAS 3355-28-0| Item | Specifications |
| Appearance | White to Off-White Powder |
| Water (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Purity/Analysis Method | ≥99.0% (HPLC) |
| Max Single Impurity | ≤0.50% |
| Residual Solvents | |
| Ethanol | ≤5000ppm |
| DMF | ≤3000ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate for Linagliptin (CAS: 668270-12-0) |
Description:
Specifications:
Package & Storage:
| Chemical Name | 8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine |
| Synonyms | 8-Bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1H-purine-2,6-dione; Linagliptin Intermediate C |
| CAS Number | 666816-98-4 |
| CAT Number | RF-PI497 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H9BrN4O2 |
| Molecular Weight | 297.11 |
| Melting Point | 285℃ |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine (CAS: 666816-98-4) is an intermediate for Linagliptin (CAS: 668270-12-0). Linagliptin is an oral, highly selective inhibitor of dipeptidyl peptidase-4 and is the first agent of its class to be eliminated predominantly via a nonrenal route. Linagliptin is indicated for once daily use for the treatment of adults with type 2 diabetes mellitus. Linagliptin was approved by the U.S. FDA in May 2011 for the treatment of Type 2 diabetes along with diet and exercise.