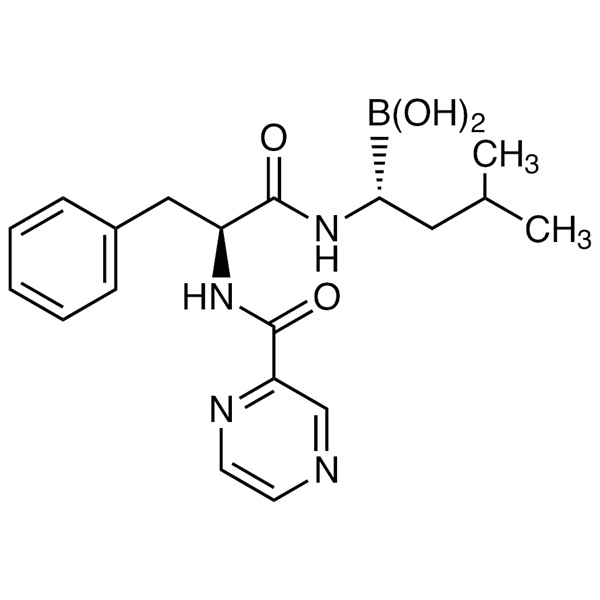
Chemical Properties:
Package: Bottle, Aluminium foil bag, Cardboard Drum, 25kg/Drum, or according to customer's requirement. Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.Supply Bortezomib Related Intermediates with High Purity (1S,2S,3R,5S)-(+)-2,3-Pinanediol CAS 18680-27-8 (1R,2R,3S,5R)-(-)-2,3-Pinanediol CAS 22422-34-0 Bortezomib CAS 179324-69-7| Item | Specifications |
| Appearance | White or Off-White Powder |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Loss on Drying | ≤0.50% |
| Residue on Ignition | ≤0.20% |
| Optical Rotation | -41.0° ~ -46.0° |
| Heavy Metal | ≤20ppm |
| Acidity | 4.0~7.0 |
| Isomer | ≤0.50% |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Residual Solvents | |
| Methanol | ≤0.30% |
| Dichloromethane | ≤0.05% |
| Hexane | ≤0.02% |
| Ethyl Acetate | ≤0.50% |
| Test Standard | Enterprise Standard |
| Usage | API |
Description:
Specifications:
Package & Storage:
| Chemical Name | Bortezomib |
| Synonyms | Velcade, MG-341, PS-341 |
| CAS Number | 179324-69-7 |
| CAT Number | RF-API65 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C19H25BN4O4 |
| Molecular Weight | 384.24 |
| Melting Point | 122.0~124.0℃ |
| Density | 1.214 |
| Refractive Index | 1.564 |
| Solubility | Soluble in Chloroform, DMSO, Ethanol and Methanol |
| Stability | Hygroscopic and Moisture Sensitive |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Bortezomib (CAS: 179324-69-7) with high quality, API. Bortezomib, sold under the brand name Velcade among others, is an anti-cancer medication used to treat multiple myeloma and mantle cell lymphoma. Bortezomib was approved for medical use in the United States in 2003 and in the European Union in 2004. It is on the World Health Organization's List of Essential Medicines. Bortezomib is the first proteasome inhibitor to be approved b the US FDA for multiple myeloma, a blood cancer. A reversible inhibitor of the 26S proteasome-a barrel-shaped multiprotein particle found in the nucleus and cytosol of all eukaryotic cells. Bortezomib is a selective and robust 26S proteasome inhibitor, that is a boronic acid dipeptide derivative. Human pancreatic cancer cell studies demonstrate Bortezomib to inhibit the PKR-like endoplasmic reticulum (ER) kinase and enhance ER stress, leading to apoptosis.