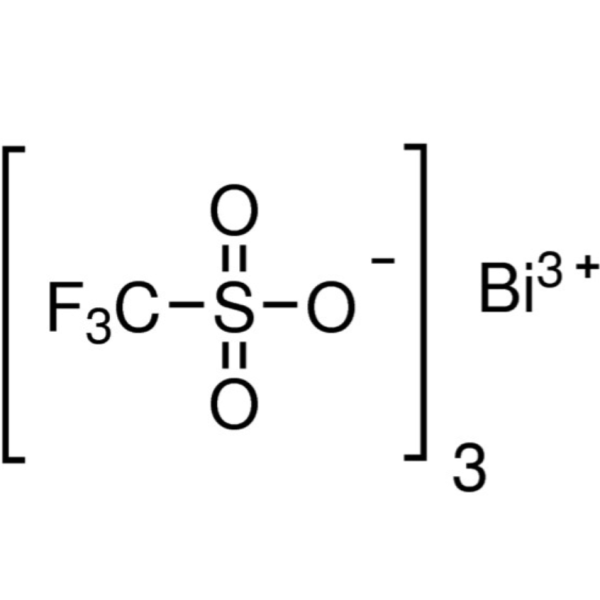
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Bismuth(III) Trifluoromethanesulfonate (CAS: 88189-03-1) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity | >98.0% |
| Bi (Complexiometric EDTA) | 31.0~32.6% |
| Fluorine NMR Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Bismuth(III) Trifluoromethanesulfonate |
| Synonyms | Bi(OTf)3; Bismuth Tris(trifluoromethanesulfonate); Bismuth(III) Triflate; Bismuth Triflate; Trifluoromethanesulfonic Acid Bismuth Salt |
| CAS Number | 88189-03-1 |
| CAT Number | RF-PI2098 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C3BiF9O9S3 |
| Molecular Weight | 656.18 |
| Melting Point | 300℃ |
| Sensitivity | Hygroscopic |
| Solubility | Soluble in Organics Acetonitrile, Dioxane, Dimethyl Formamide and Dimethyl Sulfoxide |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Bismuth(III) Trifluoromethanesulfonate (CAS: 88189-03-1) is powerful Lewis acid useful in a number of catalytic reactions. Catalyst for organic syntheses. Bismuth(III) Trifluoromethanesulfonate acts as a catalyst in Friedel-Crafts acylation and cycloisomerization of allene-enol ethers. It behaves as a direct substitution catalyst and involved in the substitution of allylic, propargylic, and benzylic alcohols with sulfonamides, carboxamides and carbamates. Further, it is also used in Mukaiyama aldol reactions. Bismuth(III) trifluoromethanesulfonate may be used as a catalyst in the following processes: deprotection of acetals; cleavage of 2-tert-butoxy derivatives of thiophenes and furans; allylation of acetals to form homoallyl ethers.