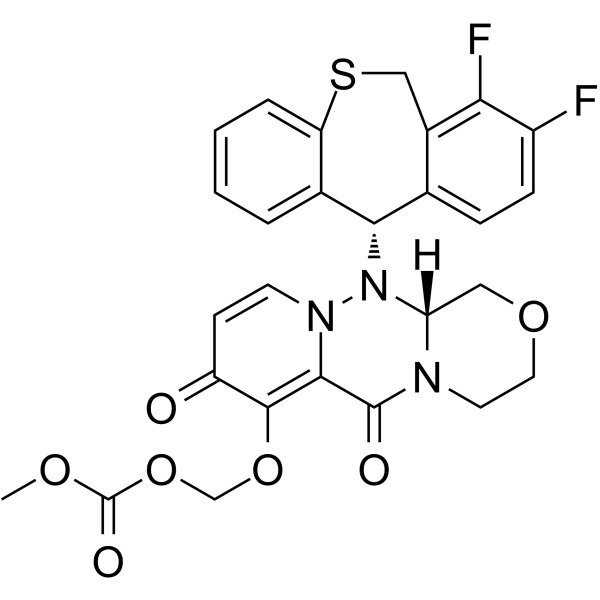
Chemical Properties:
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement. Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery. Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Baloxavir Marboxil (CAS: 1985606-14-1) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Baloxavir Marboxil, Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | White to Off-White Powder |
| Identification | The IR Spectrum corresponds to reference standard The retention time corresponds to reference standard |
| Water Content (by K.F) | ≤1.0% |
| Loss on Drying | ≤1.0% |
| Residue on Ignition | ≤0.50% |
| Heavy Metals | ≤20ppm |
| Related Substances | |
| Maximum Individual Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Particle Size | D90 Pass 150um |
| Chiral Purity | ≥99.0% |
| Purity | ≥99.0% |
| Assay | 98.0%~102.0% |
| Residual Solvents | Comply with ICH Requirements |
| Shipping | Shipping With Ice Pack |
| Test Standard | Enterprise Standard |
| Usage | API, in the treatment of Influenza A and Influenza B Infections |
Description:
Specifications:
Package & Storage:
| Chemical Name | Baloxavir Marboxil |
| Synonyms | BXM; S-033188 |
| CAS Number | 1985606-14-1 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C27H23F2N3O7S |
| Molecular Weight | 571.55 |
| Density | 1.57±0.10 g/cm3 |
| Solubility | Soluble in DMSO |
| Long-Term Storage | Store Long-term at -20℃ |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
1985606-14-1 - Application:
Baloxavir Marboxil (CAS: 1985606-14-1, BXM, S-033188, Xofluza) is an antiviral drug developed by Shionogi Co., a Japanese pharmaceutical company and Roche for the treatment of influenza A and influenza B infections. The drug was initially approved for use in Japan in February 2018 and approved by the FDA on October 24, 2018 for the treatment of acute uncomplicated influenza in patients 12 years of age and older who have been symptomatic for no more than 48 hours Label. Baloxavir marboxil, a cap-endonuclease inhibitor, has a unique mechanism of action when compared to the currently existing neuraminidase inhibitor drug class used to treat influenza infections. Baloxavir Marboxil is a new anti influenza drug with new action mechanism. Baloxavir Marboxil is the prodrug of Baloxavir Acid (S-033447) that potently and selectively inhibits the cap-dependent endonuclease within the polymerase PA subunit of influenza A and B viruses, leading to inhibition of RNA transcription and replication. Baloxavir has also been investigated for its potential to treat COVID-19 but no proven benefit has been observed.
1985606-14-1 - Mechanism of Action:
Baloxavir Marboxil is an influenza therapeutic agent, specifically, an enzyme inhibitor targeting the influenza virus' cap-dependent endonuclease activity, one of the activities of the virus polymerase complex. In particular, it inhibits a process known as cap snatching, by which the virus derives short, capped primers from host cell RNA transcripts, which it then uses for polymerase-catalyzed synthesis of its needed viral mRNAs. A polymerase subunit binds to the host pre-mRNAs at their 5' caps, then the polymerase's endonuclease activity catalyzes its cleavage "after 10–13 nucleotides". As such, its mechanism is distinct from neuraminidase inhibitors such as Oseltamivir and Zanamivir.1985606-14-1 - Pharmacokinetics:
Baloxavir Marboxil is a selective inhibitor of influenza cap-dependent endonuclease which prevents polymerase function and therefore influenza virus mRNA replication 5, 3. It has shown therapeutic activity against influenza A and B virus infections, including strains resistant to current antiviral agents 1. This drug inhibits an enzyme required for viral replication, thus rapidly treating flu virus infection 5, Label and alleviating the symptoms associated with infection. A single dose of this agent was shown to be superior to placebo in relieving influenza symptoms and superior to both oseltamivir and placebo drug in virologic outcomes (marked by decreased viral load).1985606-14-1 - Side Effects:
Common side effects following the single dose administration of baloxavir marboxil include diarrhea, bronchitis, common cold, headache, and nausea. Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of Oseltamivir.1985606-14-1 - Contraindications:
Baloxavir Marboxil should not be co-administered with dairy products, calcium-fortified beverages, or laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminum or zinc.1985606-14-1 - Uses:
Baloxavir Marboxil is an influenza medication, an antiviral,that is taken as a single dose tablet,by mouth, by individuals that are 12 years of age or older,that have presented symptoms of this infection for no more than 48 hours.The efficacy of baloxavir marboxil administered after 48 hours has not been tested.1985606-14-1 - Indications:
Baroxavir is suitable for patients ≥ 12 years old who suffer from acute uncomplicated influenza and whose symptoms do not exceed 48 hours. Attention should be paid to the limitations of medication: influenza virus changes over time, and there are factors such as virus type and subtype. Once the drug resistance of the virus and the pathogenicity of the virus change, the clinical efficacy of antiviral drugs may be weakened. When deciding whether to take basalovir dipivoxil, the available information on the sensitivity of the local epidemic virus strain to the drug should be considered.1985606-14-1 - Preparation:
Japanese patent JP6212678 reported the synthesis method of Baloxavir Marboxil. 3, 4-difluorobenzoic acid was used as raw material to react with DMF under the action of LDA to obtain 2-formyl-3, 4-difluorobenzoic acid. Then it forms thioacetal with thiophenol, then it is reduced and separated with borane to obtain 2-phenylthiomethyl -3, 4-difluorob, 8-difluorodibenzo [B, e] thiazepine -11(6H)-one, and finally the key thiazepine fragment 7, 8-difluoro-6,11-dihydrodibenzo [B, e] thiazepine -11-alcohol is obtained under the reduction of sodium borohydride. Using 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid to react with tert-butyl formate after esterification to obtain 3-(benzyloxy)-1-((tert-butoxycarbonyl) amino) -4-oxo-1, 4-dihydropyridine-2-methyl formate hydrate, and then with 2-(2, 2-dimethoxyethoxy) ethylamine undergoes urethane exchange reaction, and then cyclizes under the action of methanesulfonic acid to obtain 7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4] oxazino [3,4-c] pyridino [2,1-f][1,2,4] triazine -6, 8-dione hemihydrate, then it is condensed with (R)-tetrahydrofuran -2-formic acid, then crystallized and resolved, and then the chiral auxiliary group is removed to obtain the key chiral parent ring molecule (R)-7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4] oxazino [3,4-c] pyrido [2,1-f][1,4] Triazine-6, 8-dione. Then the key parent ring molecule is used to exchange with n-hexanol under the action of Grignard reagent, and then with the key thiazepine fragment 7,8-difluoro-6, 11-dihydrodibenzo [B, e] thiazepine -11-alcohol docking, and finally debutylation and condensation with methyl chloroformate to obtain the final product Baloxavir Marboxil.